Abstract
Background:
Stage of head and neck cancers at presentation is a strong determinant of outcomes.
Objective:
To evaluate predictors of stage of head and neck cancers at presentation and survival in a Nigerian tertiary hospital.
Patients and methods:
Health records that met the inclusion criteria for head and neck cancers were retrieved using the International Classification of Diseases, 10th revision and analyzed with associations between variables modeled using logistic regression analysis.
Results:
From a record of 487 head and neck neoplasms, 129 (26.5%) were malignant of which 122 health records met the criteria for analysis consisting of 83 (68.0%) males and 39 (32.0%) females aged 13–85 years (mean = 51 years; standard deviation = ±16 years). Alcohol (odds ratio = 1.99; 95% confidence interval = 1.08–3.69; p = 0.02) and tobacco exposure (odds ratio = 3.07; 95% confidence interval = 1.32–7.16; p = 0.01) were associated with increased odds for advanced tumor stage at presentation. Stage IV cancer (hazard ratio = 1.44; 95% confidence interval = 1.80–2.59), alcohol (hazard ratio = 2.19; 95% confidence interval = 1.18–4.10) and tobacco use (hazard ratio = 3.40; 95% confidence interval = 1.22–8.74) were associated with increased hazards for death.
Conclusion:
Alcohol, tobacco use and smoke from cooking wood are predictive factors for advanced HNC stage at presentation. Stage IV cancer, alcohol and tobacco use were associated with an increased hazard for death.
Keywords: Head and neck cancer, predictors, presentation stage, Nigeria
Introduction
The burden of cancers in sub-Saharan Africa is largely unknown and the disease continues to ravage populations in this part of the world as a result of ignorance, poverty and poor health-seeking attitudes of individuals.1
Globally, head and neck cancer accounts for more than 550,000 new cases and 380,000 deaths annually2 with two-thirds of these cases occurring in developing countries.3
They are mostly squamous cell carcinomas arising from the epithelial linings of various anatomical sites in the oral and nasal cavities, ears, pharynx, larynx, paranasal sinuses, salivary glands, facial soft tissues and the thyroid glands.4,5 Squamous cell carcinomas involving the ears are very rare compared to those in anatomic sites like the larynx.2
These tumors are known to exhibit different tumor biology, prognosis and therapeutic responses. Several risk factors are implicated in their etiology and they include tobacco and alcohol consumption; oncogenic viral infections like human papillomavirus (HPV) and Epstein–Barr virus (EBV); radiation and occupational exposures, poor socioeconomic status, race, periodontal disease and dietary factors.6,7
The major predictors of survival in head and neck cancers are early detection and the clinical stage at hospital presentation with early-stage tumors at presentation allowing for more treatment options and a resultant improvement in aesthetic and physiological functions for patients as well as a reduction in the treatment-related toxicities experienced.8 Early-stage cancers (stages I and II) have a 60%–95% chance of cure from local treatment alone while advanced cancers (stages III and IV) have more than 50% chance of recurrence and distant metastases.9 Many patients in Africa present late with clinically advanced tumors as a result of factors mentioned above,1 which result in delayed diagnosis and treatment with resultant worsening in the outcomes.10 This delay is either patient-based or healthcare provider-based and a previous report from our center stating that a delay in the availability of histological diagnosis is a negative predictor of outcomes aside late presentation.10
To the best of our knowledge, this is the first study from Nigeria aiming to describe the predictive factors of tumor stage at presentation and survival of head and neck cancers which we hope will provide information for targeting appropriate cancer education for lifestyle modification in high-risk populations and the screening of individuals for early disease detection and treatment.
Patients and methods
Study design and setting
This is a retrospective study of health records of patients with histologically confirmed head and neck cancers managed at the Jos University Teaching Hospital, Jos, Nigeria, from 1 May 2007 to 30 April 2017.
Approval for this study was provided by the Institutional Health Resource Ethics Committee (IHREC) of the hospital.
Procedure
The calculation and justification of the selected sample size for this study was done using The Survey System software package® version 11.0 (Creative Research Systems, 2016, Sebastopol, CA).
The inclusion criteria were all available complete patients’ health records of histologically confirmed head and neck cancers treated within the study period. Health records of patients who met these criteria were retrieved manually using standardized codes in the International Classification of Diseases, 10th revision (ICD-10) and the involved anatomic sites extrapolated using these codes.11 Information were obtained from patients’ records based on the comprehensive history taken by attending doctors in the Otorhinolaryngology department in the study period. The health records were cross-checked by two head and neck oncologists for reliability. Patients with incomplete health records, those who refused admission at time of presentation and whose records showed discharge against medical advice were excluded from the study.
A predesigned structured format was used to collect the data - Appendix 1.
Statistical analysis
Data generated were analyzed using EPI Info statistical software® version 7.2.2.1 (EPI Info, Centers for Disease Control and Prevention, Atlanta, GA, 2017).
The TNM staging system developed by the American Joint Committee on Cancer (AJCC) was used to stage the tumors.12 Clinical stage at presentation was the considered outcome variable (stages I–IV). The predictor variables studied were age, gender, occupation, duration of symptoms, exposure to risk factors, family history of cancer, periodontal disease and site.
The associations between variables were modeled using logistic regression analysis which was used to calculate odds ratios (ORs) and 95% confidence intervals (CIs). A p-value of ⩽0.05 was considered statistically significant. To analyze the dominant predictors of survival based on gender, alcohol and tobacco use, stage at presentation and anatomic sites and subsites, Cox proportional hazards regression analysis was used and survival analysis performed with Kaplan–Meier curves.
Results
Sociodemographic characteristics
A total of 487 cases of head and neck neoplasms (benign and malignant) were managed in the study period of which 129 (26.5%) were malignant and 122 health records met the criteria for analysis. This consisted of 83 (68.0%) males and 39 (32.0%) females giving a male-to-female ratio of 2.1:1. Patients were aged 13–85 years (mean = 51.0 years; standard deviation = ±16.0 years). The peak age incidence was in the sixth decade followed closely by the seventh decade of life (Table 1). Farmers (n = 45; 36.9%) were the commonest group of individuals who presented. Seven (5.4%) patients were excluded from the study due to incomplete health records (n = 2) and those who refused diagnosis and treatment (n = 5).
Table 1.
Sociodemographic and tumor characteristics of study population (n = 122).
| Age group (years) | Gender | Total (%) | |
|---|---|---|---|
| Male | Female | ||
| 10–19 | 2 | 0 | 2 (1.6) |
| 20–29 | 8 | 6 | 14 (11.5) |
| 30–39 | 7 | 8 | 15 (12.3) |
| 40–49 | 11 | 6 | 17 (14.0) |
| 50–59 | 22 | 9 | 31 (25.4) |
| 60–69 | 20 | 7 | 27 (22.1) |
| 70–79 | 11 | 2 | 13 (10.6) |
| 80–89 | 2 | 1 | 3 (2.5) |
| Total | 83 | 39 | 122 (100) |
| Occupation | |||
| Business | 13 | 6 | 19 (15.6) |
| Carpenter | 1 | 0 | 1 (0.8) |
| Clergy | 1 | 0 | 1 (0.8) |
| Civil servant | 9 | 5 | 14 (11.5) |
| Farmer | 43 | 2 | 45 (36.9) |
| Housewife | 0 | 24 | 24 (19.7) |
| Industrial wood worker | 1 | 0 | 1 (0.8) |
| Retired civil servant | 6 | 0 | 6 (4.9) |
| Student | 8 | 1 | 9 (7.4) |
| Tailor | 1 | 1 | 2 (1.6) |
| Total | 83 | 39 | 122 (100) |
| Primary tumor site | |||
| Nasopharynx | 24 | 10 | 34 (27.9) |
| Larynx | 18 | 7 | 25 (20.5) |
| Oropharynx | 8 | 7 | 15 (12.3) |
| Parotid | 10 | 3 | 13 (10.7) |
| Sinonasal | 18 | 10 | 28 (23.0) |
| Ext. auditory canal | 1 | 1 | 2 (1.6) |
| Parapharyngeal space | 0 | 1 | 1 (0.8) |
| Hypopharynx | 2 | 0 | 2 (1.6) |
| Facial skin | 1 | 0 | 1 (0.8) |
| Temporoparietal | 1 | 0 | 1 (0.8) |
| Total | 83 | 39 | 122 (100) |
Site and stage at presentation
The commonest tumor sites were the nasopharynx (n = 34; 27.9%), larynx (n = 25; 20.5%), sinonasal (n = 15; 12.3%) and oropharynx (n = 15; 12.3%). Other sites are shown in Table 1.
The commonest tumors in both genders were nasopharyngeal cancer, followed by laryngeal cancer.
The duration of symptoms ranged from 8 to 24 months (mean = 13.3 months; standard deviation = ±4.9).
Examination under anesthesia was done for 88 (72.1%) patients following history taking and thorough physical examination. A total of 83 (68.0%) patients had computerized tomographic scan to aid diagnosis and staging. No patient had magnetic resonance imaging (MRI) due to cost. There were 58 (47.5%) stage II disease presentations and 56 (46.0%) patients in stage III disease (Figure 1).
Figure 1.
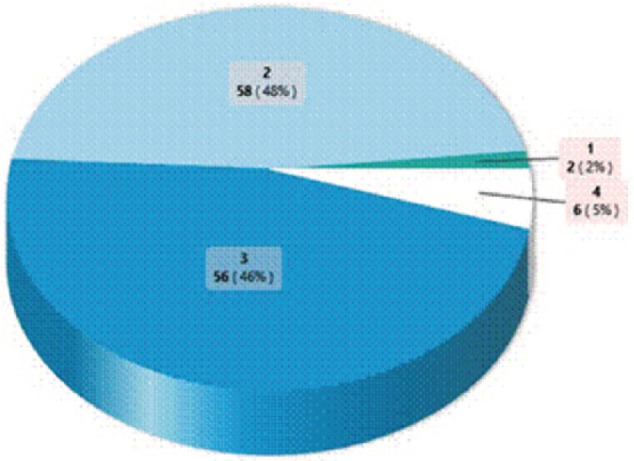
Tumor stage at presentation.
Predictors of stage
Univariate regression analysis reveals that alcohol consumption (OR = 1.99; 95% CI = 1.08–3.69; p = 0.02) and exposure to tobacco (OR = 3.07; 95% CI = 1.32–7.16; p = 0.01) were both associated with increased odds of advanced tumor stage at presentation, and concomitant alcohol and cigarette smoke exposure was also significantly associated with advanced tumor stage at presentation (OR = 1.48; 95% CI = 1.70–3.14; p < 0.001). Patients exposed to fumes from cooking wood were 3.39 times more likely to present with advanced tumors (OR = 3.39; 95% CI = 1.29–6.85; p = 0.01). Factors such as age, gender, duration of symptoms before presentation, family history of cancer and periodontal disease were not associated with advanced tumor stage at presentation (Table 2).
Table 2.
Predictive factors of tumor stage at presentation.
| Variables | Odds ratio (95% CI) | p value |
|---|---|---|
| Age | 2.05 (0.02–2.50) | 0.88 |
| Alcohol | 1.99 (1.08–3.69) | 0.02 |
| Alcohol and cigarette | 1.48 (1.70–3.14) | <0.001 |
| Cigarette | 3.07 (1.32–7.16) | <0.001 |
| Cooking wood | 3.39 (1.29–6.85) | 0.01 |
| Family history | 0.39 (0.10–1.28) | 0.11 |
| Gender | 1.00 (0.01–1.13) | 0.94 |
| Periodontal disease | 0.42 (0.01–1.20) | 0.91 |
| Symptom duration | 1.00 (0.20–4.67) | 1.00 |
| Wood dust | 1.90 (0.02–1.20) | 0.97 |
| Tumor site | ||
| Facial skin | 1.00 (1.05–1.85) | <0.001 |
| Larynx | 2.00 (1.60–11.02) | 0.001 |
| Nasopharynx | 0.40 (0.25–0.61) | 0.50 |
| Parotid | 3.23 (1.85–5.50) | 0.02 |
CI: confidence interval.
Patients with parotid gland cancers (OR = 3.23; 95% CI = 1.85–5.50; p = 0.02), laryngeal cancer (OR = 2.00; 95% CI = 1.60–11.02; p = 0.001) and facial skin cancers (OR = 1.00; 95% CI = 1.05–1.85; p < 0.001) were more likely to present at an early stage than tumors in other sites (Table 2).
Treatment
Patients had multi-modality treatment consisting of examination under anesthesia and biopsy for histological diagnosis, tracheostomy, surgical excisions with cosmetic repair and postoperative radiotherapy and/or chemotherapy. Palliation was the treatment modality for patients with stage IVC disease. A total of 102 (83.6%) patients had surgical procedures of various kinds with the commonest being maxillectomy (total and medial); 21 (17.2%) patients had emergency tracheostomy on account of upper airway obstruction while 5 (4.1%) had elective tracheostomy in consideration for airway access intraoperatively and also impending upper airway obstruction on commencement of radiotherapy.
Survival
The follow-up duration was between 5 and 68 months (median = 17.5 months; standard deviation = ±14.1) with 68 (55.7%) patients lost to follow-up.
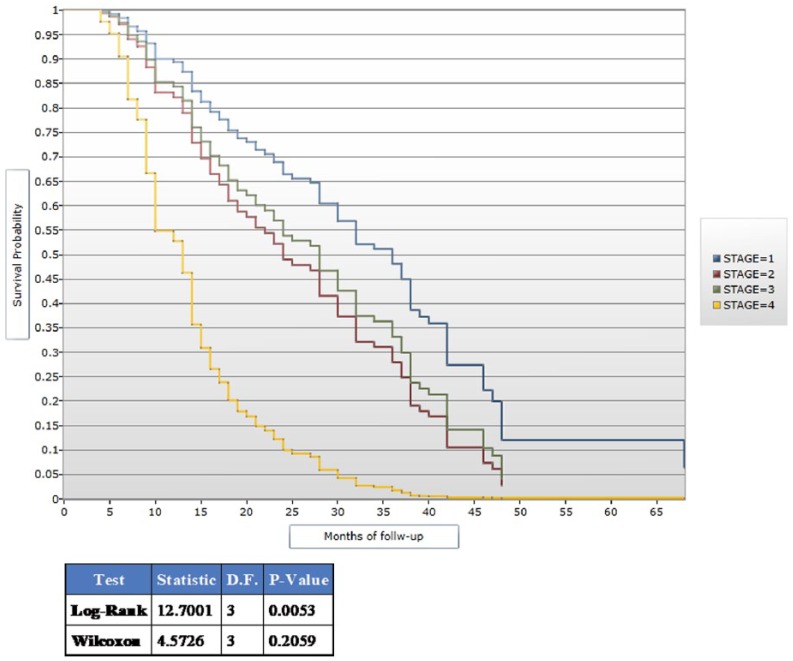
Compared to other tumor stages at presentation, patients presenting with cancer in stage IV had an increased hazard for death (hazard ratio (HR) = 1.44; 95% CI = 1.80–2.59; Figure 2). In addition, alcohol (HR = 2.19; 95% CI = 1.18–4.10) and tobacco use (HR = 3.40; 95% CI = 1.22–8.74) were associated with an increased hazard for death (Table 3).
Figure 2.
Kaplan–Meier plot showing survival by tumor stage.
Table 3.
Multivariate analysis of survival.
| Variable | Hazard ratio (95% CI) | p value |
|---|---|---|
| Gender (Male) | 1.12 (0.72–1.73) | 0.61 |
| Alcohol use | ||
| No (reference) | ||
| Yes | 2.19 (1.18–4.10) | 0.01 |
| Tobacco use | ||
| No (reference) | ||
| Yes | 3.40 (1.22–8.74) | 0.02 |
| Tumor stage | ||
| Stage I (reference) | ||
| Stage II | 1.74 (0.42–7.16) | 0.44 |
| Stage III | 1.51 (0.36–6.29) | 0.57 |
| Stage IV | 1.44 (1.80–2.59) | 0.04 |
| Tumor site | ||
| Nasopharynx | 0.44 (0.06–3.24) | 0.42 |
| Larynx | 1.50 (0.36–6.30) | 0.57 |
| Sinonasal | 1.02 (0.65–1.60) | 0.93 |
| Parotid | 0.89 (0.44–1.76) | 0.73 |
Discussion
As the overall burden of cancer is increasing in sub-Saharan Africa,13 a clear understanding of the predictive factors of HNC stage at presentation is vital for the following reasons: identifying prognosticators of disease in order to target appropriate cancer education for lifestyle modification in high-risk populations and the screening of patients for early disease detection and treatment.
The mean age at presentation in our study was 51 years and predominantly in males. This mean age is in contrast with other studies in Nigeria which report incidences in the fourth and fifth decades.14,15 Age and gender had no association with the stage of cancers at presentation in our study.
The mean time of presentation in this study was 13.3 months and was not associated with tumor stage at presentation. This non-association is similar to the work of Osazuwa-Peters et al.,16 but they, however, reported a mean time from diagnosis to initiation of treatment of 31.3 days. Late hospital presentation is a feature in our environment and is a factor that greatly influences patient outcomes.10 It is, however, interesting to note that this was not a predictive factor for tumor stage in this study. Further evaluation is required in this regard as several other studies have not been able to determine an association between tumor stage at presentation and a delay in instituting treatment.17–20 In addition to a comprehensive history of disease and a thorough examination, in order to make a diagnosis and clinically stage malignancies, investigations such as plain X-rays, computed tomography (CT) scans and MRI are employed. As a result of the high cost of these investigations and out-of-pocket payment for health services, not all patients in our study could afford CT scan with none affording MRI. Some of the stage IV cancers in our study could have been under staged as a result of this limitation.
Alcohol and tobacco use either singly or in combination were associated with a two- to threefold increased risk of presenting with advanced tumor in our study. These two risk factors act both independently and synergistically to cause genetic damage to squamous cells. Benzo[a]pyrene diol epoxide (BPDE), a known tobacco carcinogen, induces genetic damage by forming covalently bound DNA adducts throughout the genome including cytochrome p53 mutations. There are conflicting reports in the literature regarding this association with one report stating that a direct relationship exists between the use of alcohol and an advanced stage of cancer presentation,21 while another study reports no association between the stage at presentation of HNC with the use of alcohol and tobacco.22 However, a study among Black South Africans had demonstrated a major risk of laryngeal cancer in men who smoked 15 or more grams of tobacco per day.23 These two known risk factors are modifiable in the prevention of HNC as health education on discouraging their use will be of immense importance in high-risk populations.
Other established risk factors for HNC, such as positive family history of cancer, periodontal disease and exposure to wood dust,24,25 were however not associated with tumor stage at presentation in our study. However, we recorded exposure to fumes from cooking wood as being associated with advanced cancer stage at presentation especially in nasopharyngeal cancers. This is in contrast with the findings of Pacella-Norman et al.23 who reported that wood was not a significant risk factor for the cancers they studied. Wood is the available biomass fuel for cooking in low- and middle-income countries like Nigeria, and it is commonplace to cook in enclosed spaces with indoor pollution occurring from the use of these solid fuels. The International Agency for the Research on Cancer (IARC) reports an increase in the incidence and deaths from cancer as a result of carcinogens from cooking wood fumes.26 We were unable to establish the duration of exposure to this type of risk factor in our study. There are no reports in literature on the association between this exposure and tumor stage; therefore, further research is required to establish this relationship.
Our study shows that patients with parotid, laryngeal and facial skin cancers were associated with early-stage presentation as compared to tumors in other anatomical sites and subsites. This report on laryngeal cancer is similar to the reports of Osazuwa-Peters et al.16 and their reasons for the presence of a laryngeal mass causing hoarseness and especially upper airway obstruction necessitating early presentation may very well be reason for the trend noted in our study. The appearance of a mass involving the jaw or anatomical sites on the face will cause disfigurement necessitating early patient presentation and explains the early presentation noticed in patients with parotid and facial skin cancers. This is in contrast to nasopharyngeal cancers which are associated with late presentation as a result of their hidden anatomical disposition.
The relationship between exposure to HPV and EBV and tumor stage at presentation was not established in this study as our center lacks the facilities for epigenetic studies.
The attitude to follow-up is poor and a prominent feature among patients in the developing world with this attributed to poverty as patients are unable to financially keep up with hospital visits because of out-of-pocket payment for healthcare.10 All the patients from our center were referred to radio-oncologists in another health facility approximately 680 km away, a limiting factor in the effective treatment and monitoring of patients. The magnitude of loss to follow-up recorded in our study is significant enough to skew our obtained results and makes the survival curve obtained with better survival among patients with tumor stage III disease unreliable.
In accordance with a similar study,16 our study demonstrated that patients who consumed alcohol and tobacco and patients presenting with stage IV cancer had increased hazard for death.
The study had some limitations. Foremost is the fact that it is a retrospective study with poor documentation of patient health records. Second, it is from a single health institution and a small study population, factors which might have introduced selection, recall and miscalculation biases. Finally, we could not establish a relationship between viral infective agents and HNC due to non-availability of facilities for epigenetic studies. However, we were able to demonstrate the association between factors like alcohol and tobacco use, exposure to cooking wood fumes and HNC stage at presentation, providing a template for future studies on these and the association with other possible predictive factors in this part of the world.
Conclusion
The use of alcohol and tobacco and the exposure of individuals to fumes from cooking wood are predictive factors for advanced HNC stage at presentation. Stage IV cancer and alcohol and tobacco use were associated with an increased hazard for death.
We recommend a prospective community-based study to establish other predictive associations and the provision of facilities for epigenetic studies in our center to establish the relationship between infectious agents and HNC in our region.
Supplemental Material
Supplemental material, Appendix for The predictive factors of primary head and neck cancer stage at presentation and survival in a developing nation’s tertiary hospital by Adeyi A Adoga, Daniel D Kokong, Nuhu D Ma’an, Joyce G Mugu, Chukwunonso J Mgbachi and Ayuba M Dauda in SAGE Open Medicine
Acknowledgments
The authors are grateful to all the staff of the Records Department of the hospital for their invaluable assistance with patients’ health records.
Footnotes
Authors’ Contribution: A.A.A. and D.D.K. conceived the project. A.A.A., D.D.K., and N.D.M. wrote the manuscript. A.A.A., J.G.M., and C.J.M. collected the data. A.A.A., D.D.K., N.D.M., J.G.M., C.J.M., and A.M.D. analyzed and interpreted the data. All authors edited and approved the final manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from Jos University Teaching Hospital Institutional Health Research Ethical Committee (JUTH/DCS/ADM/127/XIX/6610).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Informed consent was not sought for this study because this requirement was waived by the Institutional Health Research Ethical Committee of the Jos University Teaching Hospital.
ORCID iD: Adeyi A Adoga  https://orcid.org/0000-0003-0575-5720
https://orcid.org/0000-0003-0575-5720
References
- 1. Erinoso OA, Okoturo E, Gbotolorun OM, et al. Emerging trends in the epidemiological pattern of head and neck cancers in Lagos, Nigeria. Ann Med Health Sci Res 2016; 6: 301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol 2017; 3: 524–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc 2008; 83: 489–501. [DOI] [PubMed] [Google Scholar]
- 4. Cole L, Polfus L, Peters ES. Examining the incidence of human papillomavirus-associated head and neck cancers by race and ethnicity in the U.S., 1995-2005. PLoS ONE 2012; 7: e32657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Emadzadeh M, Shahidsales S, Bajgiran AM, et al. Head and neck cancers in North-East Iran: a 25 year survey. Iran J Otorhinolaryngol 2017; 29(3): 137–145. [PMC free article] [PubMed] [Google Scholar]
- 6. Pezzuto F, Buonaguro L, Caponigro F, et al. Update on head and neck cancer: current knowledge on epidemiology, risk factors, molecular features and novel therapies. Oncology 2015; 89(3): 125–136. [DOI] [PubMed] [Google Scholar]
- 7. Adeyemi BF, Adekunle LV, Kolude BM, et al. Head and neck cancer—a clinicopathological study in a tertiary care center. J Natl Med Assoc 2008; 100(6): 690–697. [DOI] [PubMed] [Google Scholar]
- 8. Neville BW, Day TA. Oral cancer and precancerous lesions. CA Cancer J Clin 2002; 52: 195–215. [DOI] [PubMed] [Google Scholar]
- 9. Brockstein B, Haraf DJ, Rademaker AW, et al. Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: a 9-year, 337-patient, multi-institutional experience. Ann Oncol 2014; 15(8): 1179–1186. [DOI] [PubMed] [Google Scholar]
- 10. Adeyi A, Olugbenga S. The challenges of managing malignant head and neck tumors in a tropical tertiary health center in Nigeria. Pan Afr Med J 2011; 10: 31. [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization (WHO). International classification of diseases for oncology, ICD-10, 10th revision. Geneva: WHO, 2015. [Google Scholar]
- 12. Amin MB, Edge SB, Greene FL, et al. (eds). AJCC cancer staging manual. 8th ed. New York: Springer, 2017. [Google Scholar]
- 13. Morhason-Bello IO, Odedina F, Rebbeck TR, et al. Challenges and opportunities in cancer control in Africa: a perspective from the African Organisation for research and training in cancer. Lancet Oncol 2013; 14: e142–e151. [DOI] [PubMed] [Google Scholar]
- 14. Otoh EC, Johnson NW, Danfillo IS, et al. Primary head and neck cancers in north eastern Nigeria. West Afr J Med 2004; 23: 305–313. [DOI] [PubMed] [Google Scholar]
- 15. Lilly-Tariah OB, Somefun OA, Adeyemo WL. Current evidence on the burden of head and neck cancers in Nigeria. Head Neck Oncol 2009; 1: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Osazuwa-Peters N, Christopher KM, Hussaini AS, et al. Predictors of stage at presentation and outcomes of head and neck cancers in a university hospital setting. Head Neck 2016; 38(Suppl. 1): E1826–E1832. [DOI] [PubMed] [Google Scholar]
- 17. Patel UA, Brennan TE. Disparities in head and neck cancer: assessing delay in treatment initiation. Laryngoscope 2012; 122: 1756–1760. [DOI] [PubMed] [Google Scholar]
- 18. van Harten MC, Hoebers FJ, Kross KW, et al. Determinants of treatment waiting times for head and neck cancer in The Netherlands and their relation to survival. Oral Oncol 2015; 51: 272–278. [DOI] [PubMed] [Google Scholar]
- 19. Rogers SN, Pabla R, Mesorley A, et al. An assessment of deprivation as a factor in the delays in presentation, diagnosis and treatment in patients with oral and oropharyngeal squamous cell carcinoma. Oral Oncol 2007; 43: 648–655. [DOI] [PubMed] [Google Scholar]
- 20. Carvalho AL, Pintos J, Schlecht NF, et al. Predictive factors for diagnosis of advanced-stage squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg 2002; 128: 313–318. [DOI] [PubMed] [Google Scholar]
- 21. Elwood JM, Gallagher RP. Factors influencing early diagnosis of cancer of the oral cavity. Can Med Assoc J 1985; 133: 651–656. [PMC free article] [PubMed] [Google Scholar]
- 22. Scott SE, Grunfeld EA, McGurk M. Patient’s delay in oral cancer: a systematic review. Community Dent Oral Epidemiol 2006; 34: 337–343. [DOI] [PubMed] [Google Scholar]
- 23. Pacella-Norman R, Urban MI, Sitas F, et al. Risk factors for oesophageal, lung, oral and laryngeal cancers in black South Africans. Br J Cancer 2002; 86(11): 1751–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Negri E, Boffetta P, Berthiller J, et al. Family history of cancer: pooled analysis in the international head and neck cancer epidemiology consortium. Int J Cancer 2009; 124(2): 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hashim D, Sartori S, Brennan P, et al. The role of oral hygiene in head and neck cancer: results from International Head and Neck Cancer Epidemiology (INHANCE) consortium. Ann Oncol 2016; 27(8): 1619–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chafe ZA, Brauer M, Klimarot Z, et al. Household cooking with solid fuels contributes to ambient PM2.5 air pollution and the burden of disease. Environ Health Perspect 2014; 122: 1314–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Appendix for The predictive factors of primary head and neck cancer stage at presentation and survival in a developing nation’s tertiary hospital by Adeyi A Adoga, Daniel D Kokong, Nuhu D Ma’an, Joyce G Mugu, Chukwunonso J Mgbachi and Ayuba M Dauda in SAGE Open Medicine