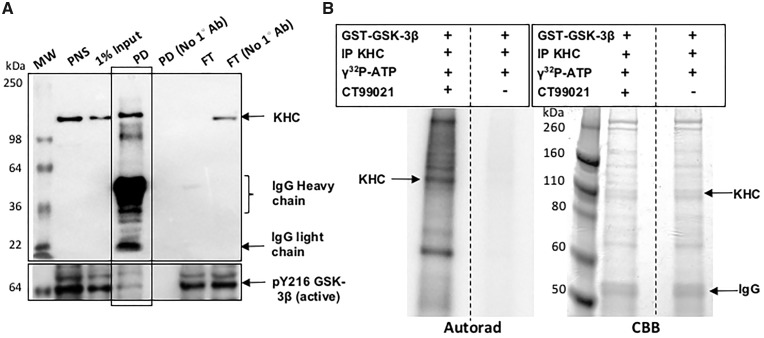
Figure 5.
Kinesin and active GSK3β coimmunoprecipitate, and KHC is phosphorylated by GSK3β in vitro. (A) Kinesin was immunoprecipitated from fly heads using a KHC antibody. In the negative control KHC antibody was not used (No 1° Ab). Western blot analysis shows a band at ∼110 kDa in the KHC pull down (PD). Note that the flow through (FT) shows no KHC band indicating that all of KHC is likely immunoprecipitated, in contrast to the no antibody FT. Active GSK3β was detected in the KHC IP by the pY216 (active) GSK3β antibody but not all active GSK3β is present with KHC. MW, molecular weight marker. (B) KHC IP was used as the substrate in the in vitro kinase assay and was incubated with GST-GSK3β in the presence of 1mCi/100 γ32P-ATP. As a control, this reaction was incubated with the GSK3β specific inhibitor CT99021. The autorad shows a strong band at ∼110 kDa indicating the phosphorylation of KHC. Note that a strong band is also observed at ∼74 kDa indicating that GSK3β is also autophosphorylated. Perhaps other proteins present in the KHC IP are also phosphorylated since all bands were absent in the reaction incubated with CT99021. However, note that the proteins are still present in both lanes in the Coomassie brilliant blue (CBB).