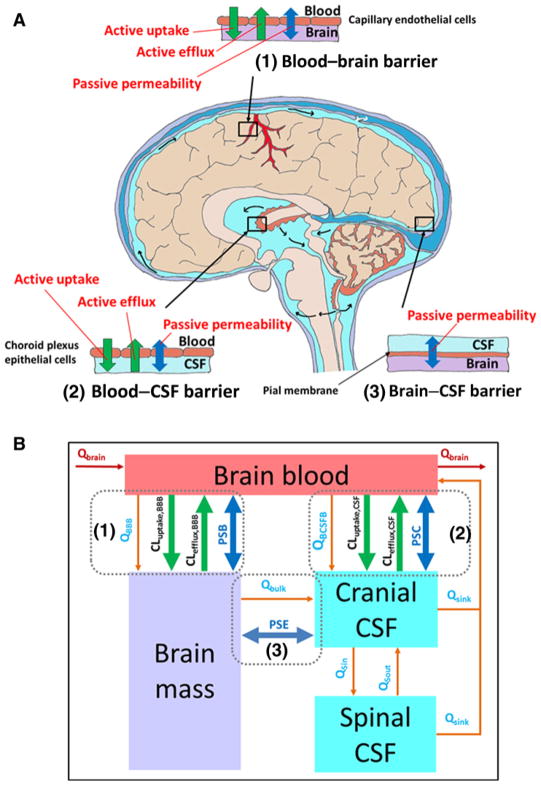
Figure 1.
A, Schematic illustration of the BBB, blood–cerebrospinal fluid (CSF) barrier, and brain–CSF barrier in human brain. Drug transport across the BBB (1) was governed by the passive bidirectional permeability and transporter-mediated active efflux and active uptake. Drug transport across the blood–CSF barrier (2) was controlled by the passive bidirectional permeability and transporter-mediated active efflux and active uptake. Drug transport across the brain–CSF barrier (3) was governed by the passive bidirectional permeability only. B, The structure of the 4Brain model as implemented in Simcyp Simulator V16. The passive bidirectional permeability across the BBB, blood–CSF barrier, and brain–CSF barrier are parameterized as PSB, PSC, and PSE, respectively. Active efflux and uptake at the BBB are parameterized as CLefflux,BBB and CLuptake,BBB, respectively. Active efflux and uptake at the blood–CSF barrier are parameterized as CLefflux,CSF and CLuptake,CSF, respectively. The model assumes that (i) only unbound and unionized drug can passively pass through all barriers, whereas transporters act upon unbound drug (including both unionized and ionized species); (ii) fluid balance is maintained by the circulation of CSF between spinal and cranial compartments and reabsorbed into the brain blood. Flow rates are described by the CSF secretion rate (QBCSFB), bulk flow rate from brain mass to cranial CSF (Qbulk), CSF flow rate out of cranial and spinal compartments (Qsink), CSF shuttle flow rate between cranial and spinal compartments (QSin and QSout), water transfer rate from the brain blood to brain mass (QBBB); (iii) the cerebral blood flow rate (QBrain) links the brain model to the whole-body PBPK model; (iv) metabolism in brain mass is negligible; and (v) all compartments are well stirred with defined volumes.