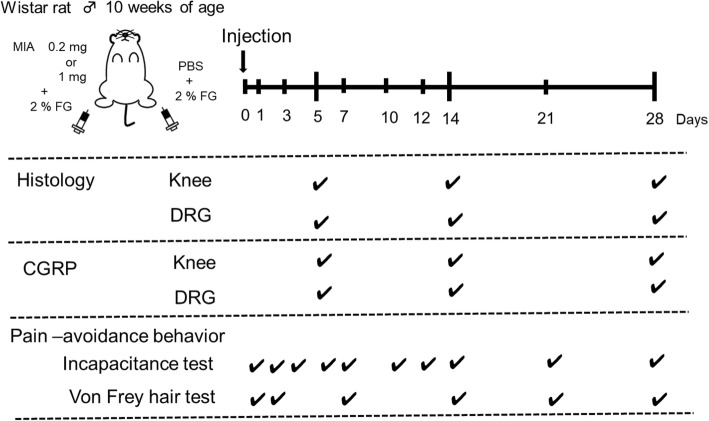
Fig. 1.
Study design. The right knee joint had an intra-articular injection of MIA at day 0. The left knee had PBS as a control. Histological and immunohistochemical evaluations (hematoxylin and eosin/safranin O staining and calcitonin gene-related peptide staining) were performed at 5, 14, and 28 days post-injection. The pain avoidance behavior tests (incapacitance and von Frey) were performed as indicated