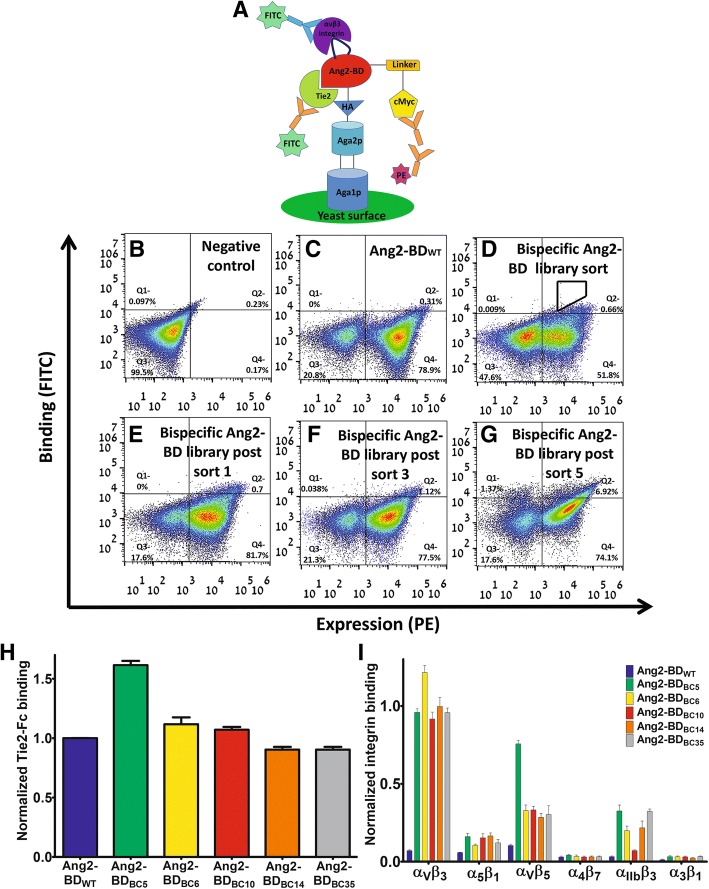
Fig. 1.
Affinity maturation of the Ang2-BDRGD-based library bi-specific for αvβ3 integrin and Tie2-Fc. a Ang2-BD was presented on the yeast cell surface as a fusion with agglutinin proteins. Display levels were detected using primary antibodies against the C-terminal cMyc tag (chicken anti-cMyc antibodies) and phycoerythrin (PE)-conjugated anti-chicken antibodies. Binding to Tie2-Fc was determined using fluorescein isothiocyanate (FITC)-conjugated anti-human Fc antibodies. Binding to αvβ3 integrin was determined using FITC-labeled mouse anti-αv integrin antibodies. b–g FACS analysis of the binding of the bi-specific Ang2-BD-based library to αvβ3 integrin in different screening steps. Quadrant gate statistics are indicated in each panel b negative control. c Ang2-BDWT expression and αvβ3 integrin binding (10 nM). d Expression of the bi-specific Ang2-BDRGD-based library and αvβ3 integrin binding (10 nM) at pre-sorting and e–g expression of the bi-specific Ang2-BD-based library and αvβ3 integrin binding (10 nM) after sorts 1, 3, and 5, respectively. h Binding of isolated yeast-displayed bi-specific Ang2-BDRGD clones to Tie2 (20 nM). Data were normalized to the yeast surface expression levels of each clone and Tie2 binding of Ang2-BDWT. i Binding of isolated yeast-displayed bi-specific Ang2-BDRGD clones to αvβ3, α5β1, αvβ5, α4β7, αIIbβ3, and α3β1 integrins (50 nM). Data were normalized to the yeast surface expression level of each clone. Data shown represent the average of triplicates from independent experiments, and error bars represent the standard error of the mean