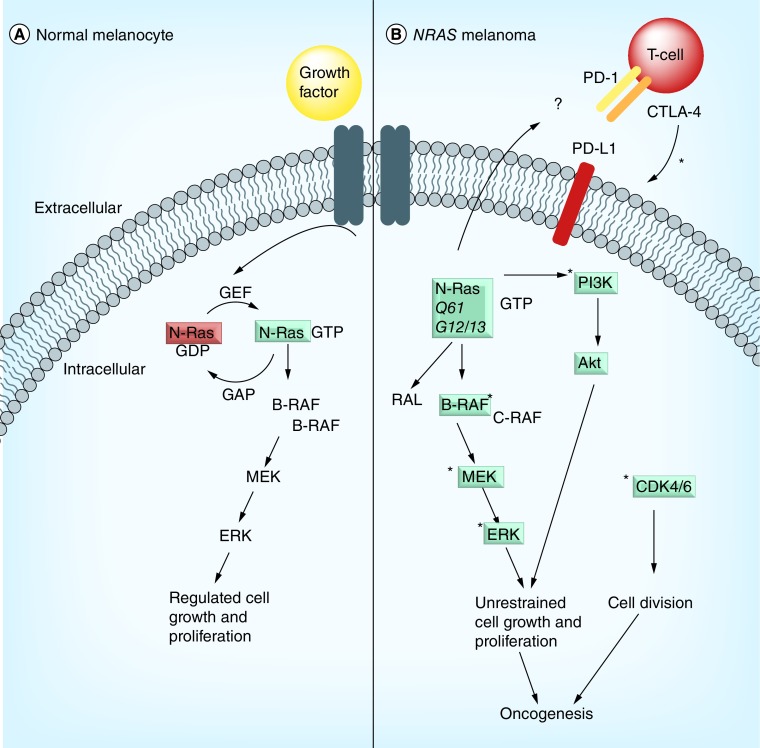
Figure 1. . N-Ras signaling and effector pathways.
(A) In normal melanocytes, N-Ras is inactive until a stimulus (e.g., growth factor) binds its receptor tyrosine kinase, which promotes exchange of GDP to GTP by GEFs, thereby activating N-Ras. In normal melanocytes, activation of N-Ras signaling is mediated by the Raf-MEK-ERK pathway. (B) In NRAS mutant melanoma, the N-ras protein is unable to hydrolyze GTP to GDP, and remains in the GTP-bound, active state. Oncogenic N-Ras stimulates various Ras effector pathway, including Raf, PI3K/Akt, and RAL. Ras-Raf-MEK-ERK signaling drives cellular proliferation, as does the PI3K/Akt pathway. Downstream signaling can be disrupted via inhibition of MEK signaling, and in cooperation with MEK inhibition. Disruption of CDK4/6 signaling cooperates to disable tumor growth in NRAS mutant melanoma. There is some suggestion the response to immune checkpoint blockade is enhanced in patients with NRAS mutant melanoma, but the reports are limited and a proposed mechanism is lacking. Treatment of NRAS mutant melanoma (*) involves targeting MEK, PI3K/Akt, CDK4/6, or immune checkpoints (PD1, CTLA-4).