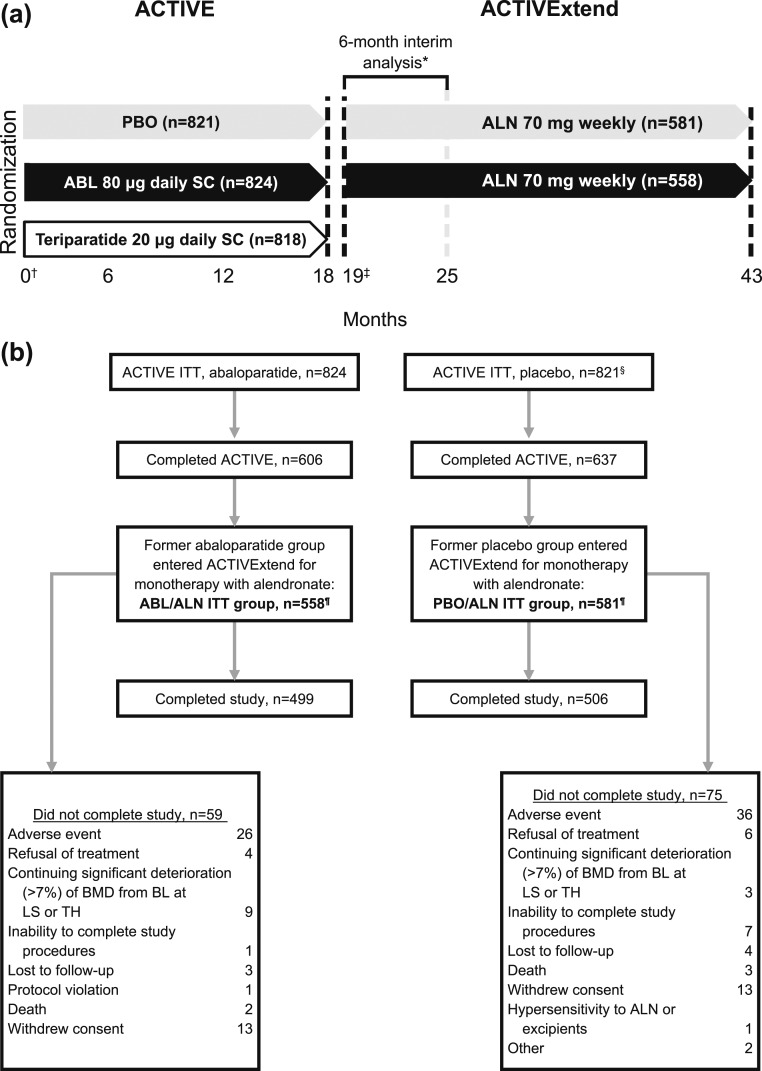
Figure 1.
Study design and participant enrollment and disposition. (a) Study design. Participants were treated with ABL, teriparatide, or PBO for 18 mo in ACTIVE. Participants who received ABL or PBO during ACTIVE were eligible for enrollment in ACTIVExtend. A gap in treatment of up to 1 mo (from mo 18 to 19) was allowed for rollover and reconsenting from ACTIVE to ACTIVExtend. Former ABL and former PBO participants began monotherapy with ALN at cumulative mo 19 for up to 24 mo. (b) Participant enrollment and disposition. *As reported by Cosman et al. (10). †Baseline for the integrated 43-mo analysis was day 1 of ACTIVE. ‡Baseline of the ACTIVExtend analysis was day 1 of ACTIVExtend (cumulative mo 19). §As reported by Miller et al. (9). ¶A modified intent-to-treat (mITT) population, including those participants with an evaluable postbaseline spine radiograph assessment, was evaluated for incident new vertebral fractures. Fourteen ABL/ALN participants (one because she was not included in the mITT population for ACTIVE and 13 because they did not have a postbaseline spinal radiograph) were not included in the ACTIVExtend mITT, yielding an ABL/ALN mITT of n = 544; 13 PBO/ALN participants (two because they were not included in the ACTIVE mITT and 11 for no postbaseline spinal radiograph) were excluded from the mITT, yielding an mITT n = 568. BL, baseline.