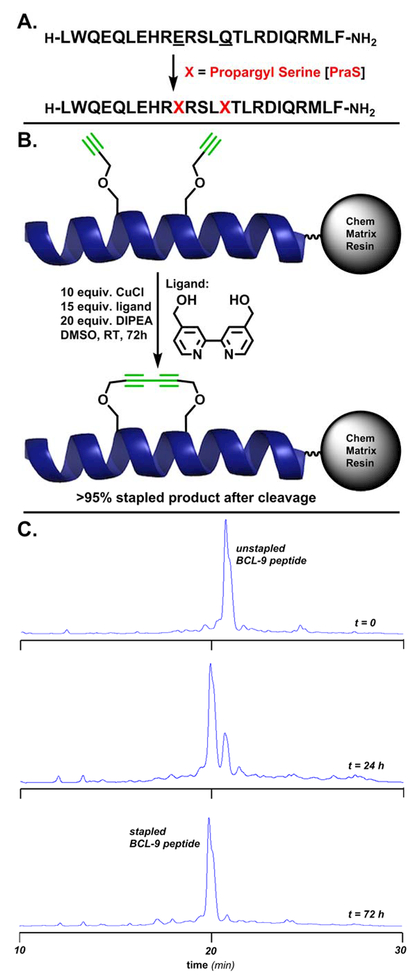
Figure 2.
A. The BCL9 24-mer peptide. Underlined residues were chosen for substitution to propargyl serine to form the i, i+4 diyne staple. The peptide was synthesized on Rink Amide ChemMatrix resin. B. Reaction conditions for on-resin Glaser stapling. The displayed helix is the wild-type BCL9 24-mer, PDB entry 2GL7. C. From top to bottom, HPLC traces of crude peptide cleaved in 10% DMS, 2.5% TIPS, 2.5% H2O cocktail (2 hours) after Glaser stapling reaction at t=0, t=24 (76% conversion by peak area), and t=72 hrs (>95% conversion by peak area).