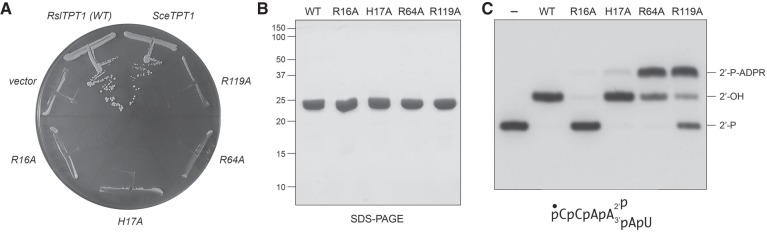
FIGURE 5.
Four conserved amino acids are essential for RslTpt1 activity in vivo. (A) Test of RslTPT1-Ala mutants for S. cerevsiae tpt1Δ complementation by plasmid shuffle. Yeast tpt1Δ p[CEN URA3 TPT1] cells were transformed with CEN plasmids bearing wild-type SceTPT1 (positive control), empty CEN vector (negative control), and wild-type RslTPT1 or the indicated RslTPT1-Ala mutant alelles. Single transformants were selected and streaked on agar medium containing 5-FOA. The plate was photographed after incubation for 7 d at 37°C. (B) Aliquots (5 µg) of recombinant RslTpt1 were analyzed by SDS-PAGE. The Coomassie blue-stained gel is shown. The positions and sizes (kDa) of marker polypeptides are indicated on the left. (C) Reaction mixtures (10 µL) containing 100 mM Tris-HCl (pH 7.5), 5 mM MgCl2, 1 mM NAD+, 0.1 µM (1 pmol) 5′ 32P-labeled 6-mer 2′-PO4 RNA, and 0.05 µM (0.5 pmol) RslTpt1 (wild-type or mutant) were incubated at 37°C for 30 min, then mixed with an equal volume of 90% formamide, 50 mM EDTA. The products were resolved by urea-PAGE and visualized by autoradiography.