Fig 3. Addition of N-linked oligosaccharide at fourth position does not change efficiency of amino-terminal tripeptide-enhanced GH trafficking.
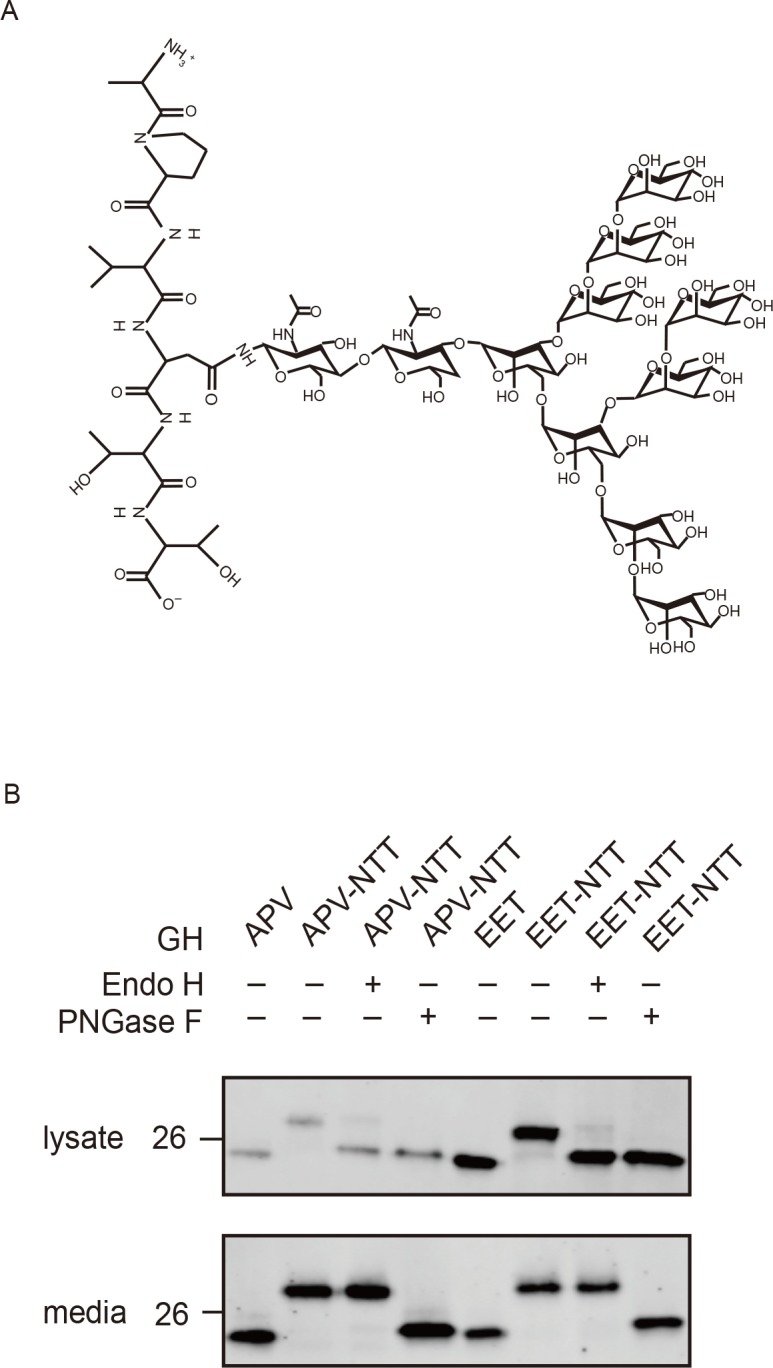
(A) Chemical structure of N-linked glycosylation on APVNTT peptide (amino-terminal alanine at top). (B) Western blot: GH starting with high-efficiency motif, APV, obtained lower steady-state levels in HEK293A cells compared to same protein starting with predicted nonbinding motif, EET. Addition of N-linked oligosaccharide (NTT motif inserted at position 4) caused PNGaseF-susceptible increases in size (Mr) but did not change relative trafficking efficiencies of either strong (APV-NTT) or nonbinding (EET-NTT) proteins. The oligosaccharides on cell layer–associated GH by both constructs were removed by Endo H digestion, showing no Golgi modifications. Cells and media were collected 16 hr posttransfection. Three μg of cell lysate or 6% of concentrated media was used for western blot analysis with goat anti-human GH. LI-COR IR-fluorescent second antibodies were used for detection on LI-COR’s Odyssey scanner. Numbers on left are molecular weight standards in kDa. APV, alanine-proline-valine; EET, glutamic acid–glutamic acid–threonine; GH, growth hormone; HEK293A, human embryonic kidney cell line 293A; IR, infrared; NTT, asparagine-threonine-threonine.
