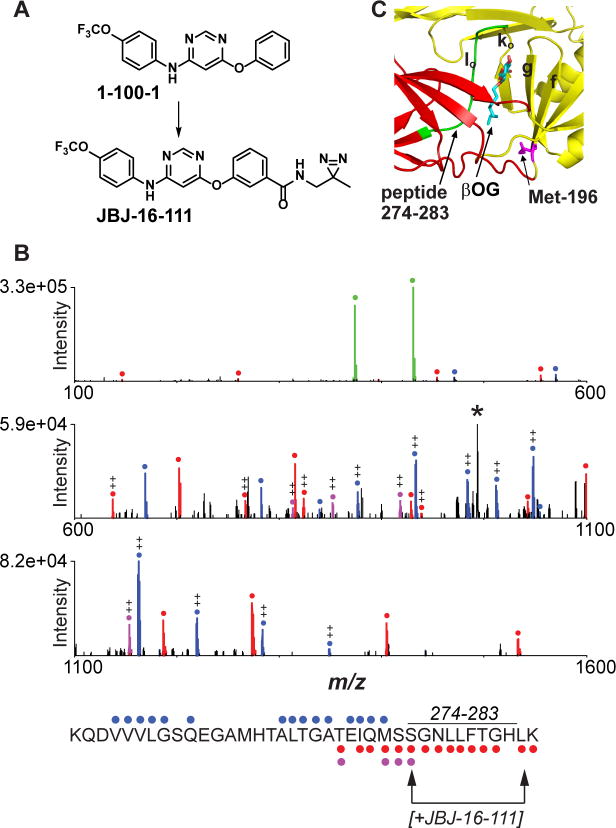
Figure 3. Photocrosslinking of a 4,6-disubstituted inhibitor in the βOG pocket.
(A) Structures of inhibitor JBJ-16-111 and parental compound 1-100-1. (B) HCD MS/MS spectrum of the peptide KQDVVVLGSQEGAMHTALTGATEIQMSSGNLLFTGHLK (residues 247-284) acquired during CE-MS analysis of tryptic peptides from the UV crosslinking reaction between DI,II protein and JBJ-16-111. Unmodified b- and y- type ions are marked with blue and red circles, respectively, while y-ions modified by JBJ-16-111 are highlighted in purple. Abundant probe-related ions at m/z 431.12 and 374.07 (green), along with neutral-loss ion at m/z 993.24 (denoted ‘*’), suggest that JBJ-16-111 is readily lost during MS/MS. Fragment ion evidence suggests that the peptide is labeled between S274 and L283 (see arrows; assumes C-terminal lysine is not modified due to successful trypsin cleavage). ++, doubly charged ion. (C) DENV2 E structure (1OKE) with residues 274-283 highlighted in green.