Table 1.
Reaction Parameters for Annulations to Azolotriazines 6a
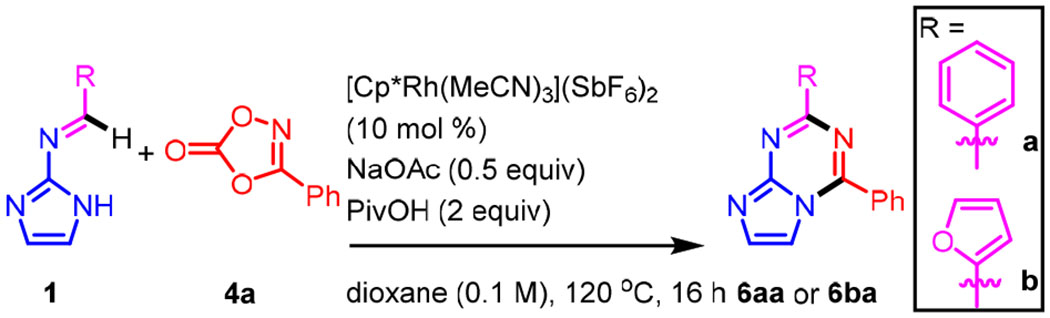
| entry | imine | variation | Yield %b |
|---|---|---|---|
| 1 | 1a | none | 75 (73)c |
| 2 | 1a | [Cp*RhCl2]2, AgSbF6d | 60 |
| 3 | 1a | [Cp*RhCl2]2(5 mol %) | 8 |
| 4 | 1a | no Rh | 0 |
| 5 | 1a | no PivOH | 12 |
| 6 | 1a | no NaOAc | 16 |
| 7 | 1a | NaOAc (1 equiv) | 79 |
| 8 | 1a | 100 oC | 70 |
| 9 | 1a | 0.2 M | 46 |
| 10 | 1a | toluene as solvent | 11 |
| 11 | 1a | DCE as solvent | 9 |
| 12 | 1a | MeCN as solvent | 25 |
| 13 | 1a | [Cp*IrCl2]2, AgSbF6d | 10 |
| 14 | 1a | [Cp*Co(MeCN)3](SbF6)2 | 0 |
| 15 | 1b | none | 53 (55)e |
| 16 | 1b | NaOAc (1 equiv) | 37 |
| 17 | 1b | 100 °C | 43 |
