Abstract
Objectives
To determine the association of the frailty phenotype with subsequent health care costs and utilization.
Design
Prospective cohort study (Study of Osteoporotic Fractures [SOF]).
Setting
Four U.S. sites.
Participants
2,150 community-dwelling women (mean age 80.2 years) participating in SOF Year 10 (Y10) examination linked with their Medicare claims data.
Measurements
At Y10, frailty phenotype defined using criteria similar to those used in the Cardiovascular Health Study frailty phenotype and categorized (robust, intermediate stage or frail). Participant multimorbidity burden ascertained using claims data. Functional limitations assessed by asking about difficulty performing instrumental activities of daily living. Total direct health care costs and utilization ascertained during 12 months following Y10.
Results
Mean (SDs) of total annualized costs (2014 dollars) were $3,781 (6,920) among robust, $6,632 (12,452) among intermediate stage and $10,755 (16,589) among frail women. After adjustment for age, site, multimorbidity burden and cognition, frail vs. robust women had greater mean total costs (cost ratio [CR] 1.91 [95% CI 1.59-2.31]), outpatient costs (CR 1.55 [95% CI 1.36-1.78]) and higher odds of hospitalization (odds ratio [OR] 2.05 [95% CI 1.47-2.87]) and skilled nursing facility stay (OR 3.85 [95% CI 1.88-7.88]). Smaller but significant effects of the intermediate stage category on these outcomes were present. Individual frailty components (shrinking, poor energy, slowness and low physical activity) were also each associated with higher total costs. The association of the frailty phenotype with total costs was partially mediated by functional limitations (CR further adjusted for self-reported limitations 1.32 [95% CI 1.07-1.63] among frail vs. robust and 1.35 [95% CI 1.18-1.55] among intermediate stage vs. robust women).
Conclusions
Intermediate stage and frail older community-dwelling women had higher subsequent total health care costs and utilization after accounting for multimorbidity and functional limitations. Frailty phenotype assessment may improve identification of older adults likely to require costly, extensive care.
Keywords: frailty, multimoribidity, health care utilization, health care costs
INTRODUCTION
Older age and multiple chronic medical conditions (multimorbidity) among Medicare beneficiaries in the United States (US) are associated with higher health care costs and greater utilization.1;2 However, there is considerable individual variability in health care costs and utilization in aged populations and only a modest proportion of the variance in total health care costs is explained by multimorbidity.3 Hence, more accurate identification of older patients who are likely to require intense, costly care is needed.
Frailty, a geriatric syndrome of reduced reserves and decreased resistance to stressors resulting from cumulative decline across multiple physiologic systems, has been associated with an increased risk of adverse health outcomes. In an attempt to operationalize the syndrome of frailty, Fried and colleagues4 using data from the Cardiovascular Health Study (CHS) proposed a phenotype of frailty in which 3 or more of the following 5 components were present: shrinking, weakness, poor energy, slowness and low activity level. Although operational definitions of frailty vary, the validity of the CHS frailty phenotype5-7 for prediction of risk of adverse clinical outcomes has been confirmed in several cohorts.
A cross-sectional study of older German adults8 reported that frailty as defined by a modified CHS phenotype9 was associated with higher health care costs, but relied on patient recall to derive estimates of costs. Most studies examining the association of the frailty phenotype with health care utilization focused on past hospitalization4;10 or enrolled selected patient populations.11;12
To examine the association of the frailty phenotype with subsequent total direct health care costs and utilization, we used a unique longitudinal data set comprised of 2,150 women (mean aged 80.2 years) participating in the Year 10 (Y10) examination of the Study of Osteoporotic Fractures who were linked to their Medicare claims data.
METHODS
Study Population and Linkage to Inpatient Claims
We studied participants enrolled in the Study of Osteoporotic Fractures (SOF), a prospective cohort study of community-dwelling women. From 1986-88, 9,704 Caucasian women ≥65 years old and able to walk unassisted were recruited for participation from 4 geographic areas of the United States.13 Linkage of cohort data and Medicare claims was successful for 8,604 women (92.3 % of surviving participants) as of 1/1/1991, the earliest data for which outpatient Medicare claims are available.14;15 There were 5,042 women from the original cohort who completed a Y10 examination (clinic or home) that included assessment of frailty phenotype (Supplementary Figure). Of these women, the analytic cohort included 2,150 women who had data for the frailty phenotype and were also enrolled in the Medicare Fee-For-Service (FFS) program (Parts A and B [and not Part C, Medicare Advantage]) from 12 months prior to the Y10 examination until 12 months following the Y10 examination (or up until death within this period).
Measurements
Participants were asked about education and smoking status. Physical activity (expressed as a weighted score of kilocalories/week) was assessed using a modified version of the Harvard Alumni Questionnaire.16 Depressive symptoms were evaluated using the 15-item Geriatric Depression Scale17 (which includes the question “Do you feel full of energy?”). Cognitive function was assessed with a modified version of the Mini-Mental State Examination18 with a maximum score of 26. Tests of physical performance included grip strength (handheld dynamometer) and usual gait speed (average speed at usual pace in 2 trials over a 6 m course). Body weight and height were measured and body mass index was calculated. Women were asked if they had difficulty performing any of 5 instrumental activities of daily living (IADL).19
Participant multimorbidity burden was ascertained with a count of Elixhauser conditions20, a sum of indicator variables for 31 specific medical conditions derived from ICD9 codes in Medicare inpatient and outpatient claims during the 12 months prior to the Y10 SOF examination. Multimorbidity was categorized as none (0-1 condition), mild (2-3 conditions) or at least moderate (≥4 conditions).
Frailty Phenotype
The frailty phenotype was operationalized using criteria similar to those of the CHS frailty phenotype4:
Shrinking: weight loss of ≥5% between the Y8 and Y10 examinations (mean 2.3 years between examinations)
Weakness: Y10 grip strength in the lowest quintile stratified by body mass index (quartiles).
Poor energy: answered “no” to the question “Do you feel full of energy?” on the Y10 Geriatric Depression Scale.
Slowness: Y10 gait speed in the lowest quintile stratified by standing height (median).
Low physical activity: Y10 weighted score of kilocalories expended per week in the lowest quintile.
Women were robust if no components were present, intermediate stage (i.e. pre-frail) if 1 or 2 components were present, and frail if ≥3 components were present.
Outcome Measures
The primary outcome was total direct health care costs for the 12 months following the Y10 examination. We used 12 months cost data for everyone, including the 44 women (2.0%) who died during this time period. Total costs were calculated as the sum of costs for acute hospital stays, skilled nursing facility (SNF) stays paid under Medicare part A, inpatient rehabilitation facility (IRF) stays, outpatient care, and home health care for that time period. Acute hospital stays, SNF stays, and IRF stays were identified in the Medical Provider Analysis and Review (MedPAR) file. Standardized costs for acute short hospital stays, SNF stays and IRF stays were estimated using a previously published and validated method.15;21 Costs for outpatient utilization and home health care utilization were based on the allowable charges for these services in the Carrier, Outpatient and Home Health Care Medicare claims files.22 The costs of all units of utilization were adjusted for health care cost inflation to U.S. 2014 dollars.15
Statistical Analysis
Generalized linear models (GLMs) were used to estimate the association of the Y10 frailty phenotype with mean total direct health care costs over the subsequent 12 months. Gamma distributions with log links were chosen based on Modified Park23 and Pregibon link tests.24 GLMs (gamma distributions, log links) were also used to analyze the association of the frailty phenotype with mean outpatient costs. Logistic models were used to estimate the associations of the frailty phenotype with risks of ≥1 hospitalization and ≥1 SNF stay. Robust women were the referent group. In addition, GLMs (gamma distributions, log links) were used to determine the association of each frailty component with subsequent total health care costs.
Base models included age and study enrollment site. Potential confounders (Table 1) were screened for inclusion in multivariable models. Candidate covariates were included in multivariable models as confounders if they were associated with frailty phenotype; were independently related to total health care costs after adjustment for age, site and frailty phenotype; and were not utilized to define a component of the frailty phenotype. Initially, multivariable models were further adjusted for multimorbidity and cognitive function. To determine if the association of the frailty phenotype with outcomes was explained by greater self-reported functional limitations among intermediate stage and frail participants, IADL impairment was then added to multivariable models.
Table 1.
Characteristics of Participants by Category of Frailty Phenotype
| Overall | Robust | Intermediate Stage | Frail | ||
|---|---|---|---|---|---|
| Characteristica | N=2150 | N=554 | N=1188 | N=408 | P |
| Age, years, mean (SD) | 80.2 (4.4) | 78.8 (3.6) | 80.1 (4.1) | 82.6 (5.1) | <0.001 |
| Ever smoker, n (%) | 857 (39.9) | 205 (37.0) | 479 (40.3) | 173 (42.4) | 0.21 |
| Education, years, mean (SD) | 12.8 (2.8) | 12.9 (2.9) | 12.9 (2.7) | 12.4 (2.8) | 0.011 |
| Multimorbidityb, n (%) | <0.001 | ||||
| None (0-1 conditions) | 862 (40.1) | 295 (53.2) | 480 (40.4) | 87 (21.3) | |
| Mild (2-3 conditions) | 776 (36.1) | 190 (34.1) | 443 (37.3) | 143 (35.0) | |
| At least moderate (≥4 conditions) | 512 (23.8) | 69 (12.5) | 265 (22.3) | 178 (43.6) | |
| GDS score (0-15), mean (SD) | 2.4 (2.8) | 0.6 (1.4) | 2.6 (2.7) | 4.4 (3.1) | <0.001 |
| MMSE score (0-26), mean (SD) | 23.8 (2.4) | 24.1 (2.0) | 23.9 (2.3) | 23.0 (3.2) | <0.001 |
| IADL impairments (0-5), mean (SD) | 1.1 (1.5) | 0.2 (0.6) | 0.9 (1.3) | 2.6 (1.7) | <0.001 |
| Body mass index, mean (SD) | 26.6 (4.9) | 26.1 (4.2) | 26.8 (5.1) | 26.5 (5.3) | 0.02 |
| Total annual health care costs, USD | |||||
| Median (IQR) | $1,892 (677-6,533) | $1,343 (494-3,217) | $1,953 (741-6,459) | $3,640 (1001-13,189) | |
| Mean (SD) | $6,680 (12,466) | $3,781 (6,920) | $6,632 (12,452) | $10,755 (16,589) | <0.001 |
| Incident hospitalization, n (%) | 534 (24.8) | 89 (16.1) | 287 (24.2) | 158 (38.7) | <0.001 |
| Incident SNF stay, n (%) | 133 (6.2) | 11 (2.0) | 72 (6.1) | 50 (12.3) | <0.001 |
| Annual outpatient costs, USD | <0.001 | ||||
| Median (IQR) | $1,465 (634-2,935) | $1,121 (481-2,185) | $1,520 (672-3,063) | $1,826 (778-3,407) | |
| Mean (SD) | $2,337 (3,408) | $1,656 (1,714) | $2,449 (3,414) | $2,937 (4,724) | |
| Died during follow-up, n (%) | 44 (2.0) | 4 (0.7) | 15 (1.3) | 25 (6.1) | <0.001 |
Note: GDS, Geriatric Depression Scale; MMSE, Mini Mental State Examination; IADL, instrumental activities of daily living; USD, U.S. 2014 dollars; SNF, skilled nursing facility; IQR, interquartile range
Participant characteristics assessed at SOF Year 10 examination; total costs, hospitalizations, SNF stays, and outpatient care costs ascertained during 12 month period after SOF year 10 examination
Multimorbidity quantified using diagnoses in inpatient and outpatient claims data
RESULTS
The study cohort at Y10 included 2,150 community-dwelling women with mean (SD) age of 80.2 (4.4) years (Table 1). Among these women, 408 (19.0%) were frail, 1,188 (55.3%) were intermediate stage, and 554 (25.8%) were robust. There were 1,288 women (59.9%) with at least mild multimorbidity and 512 (23.8%) with at least moderate multimorbidity. Frail women had a greater burden of co-existing medical conditions; at least moderate multimorbidity was noted among 12.5% of robust women vs. 43.6% of frail women. However, some variability in multimorbidity was present within each frailty phenotype category. For example, 21.3% of frail women had no multimorbidity, while 12.4% of robust women had at least moderate multimorbidity. Self-reported functional limitations also varied across the frailty phenotype; mean number of IADL impairments was 0.2 among robust women increasing to 2.6 among frail women.
Among the overall cohort during the 12 months following Y10, the median (interquartile range [IQR]) total health care costs (2014 U.S. dollars) were $1,892 (677-6,533) and mean (SD) costs were $6,680 (12,466). A total of 534 women (24.8%) had at least 1 hospitalization and 133 (6.2%) had at least 1 SNF stay.
Characteristics (including the distribution of the frailty phenotype) of the 2,150 women in the analytical cohort were similar to those of the 2,892 SOF women attending Y10 examination who were excluded from analyses because they were not enrolled in a FFS plan (Supplementary Table 1).
Association of the Frailty Phenotype with Total and Outpatient Health Care Costs
Mean and median total health care costs in the 12 months after Y10 exam were higher with greater degree of frailty at Y10 (Table 1). Mean (SD) costs increased from $3,781 (6,920) among robust women to $6,632 (12,452) among intermediate stage women to $10,755 (16,589) among frail women (p<0.001). Greater degree of frailty was also associated with a higher likelihood of incurring very high costs; the proportion of women with costs in the highest decile ($19.319-$122,776) was 4.0% among robust women, 9.9% among intermediate stage women and 18.6% among frail women (p<0.001) (Figure 1).
Figure 1.
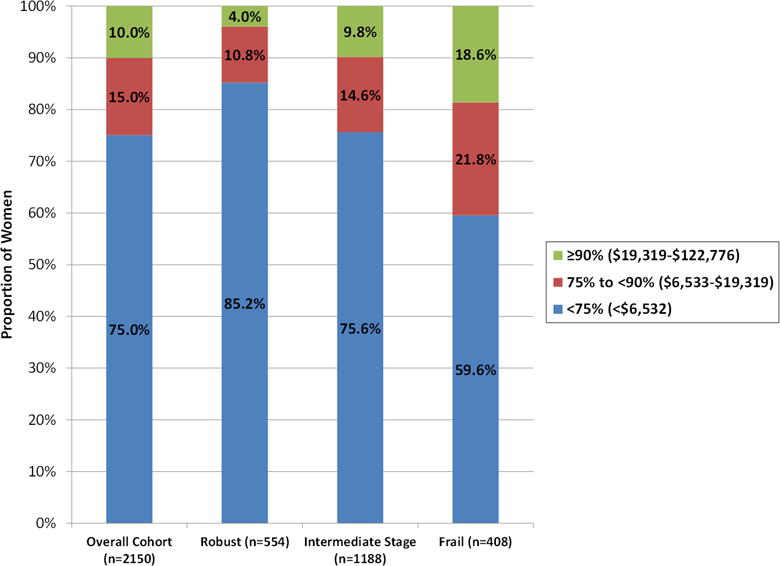
Distribution of Total Health Care Costs Overall and by Category of Frailty Phenotype
After adjustment for age and study site, mean total costs were 2.6-fold higher (cost ratio [CR] 2.61 [95% CI 2.19-3.12]) among frail vs. robust women and 1.7-fold higher (CR 1.66 [95% CI 1.45-1.91]) among intermediate stage vs. robust women (Table 2). After further accounting for multimorbidity and cognitive function, these associations were somewhat attenuated but remained significant (CR 1.91 [95% CI 1.59-2.31] among frail women and 1.50 [95% CI 1.31-1.72] among intermediate stage women). The associations of intermediate stage and frailty with higher total costs were mediated at least in part by the poorer functional status of women with these phenotypes. After additional adjustment for functional limitations, costs were approximately 1.3-fold higher among both groups (CR 1.32 [95% CI 1.07-1.63] among frail women and 1.35 [95% CI 1.18-1.55] among intermediate stage women). In comparison, mean total costs in the fully adjusted model were 1.2-fold higher (CR 1.20 [95% CI 1.05-1.36]) among women with mild multimorbidity and 1.9-fold higher (CR 1.87 [95% CI 1.60-2.18]) among women with at least moderate multimorbidity vs. women without multimorbidity. Each additional IADL impairment in the fully adjusted model was associated with a 1.2-fold increment in mean total costs (CR 1.19 [95% CI 1.13-1.25]).
Table 2.
Association of Frailty Phenotype with Mean Total and Outpatient Health Care Costs
| Total health care costs | Outpatient costs | |||
|---|---|---|---|---|
| Frailty phenotype | Cost ratio (95% CI) | P | Cost ratio (95% CI) | P |
| Base modela | ||||
| Robust | 1.00 (referent) | <0.001 | 1.00 (referent) | <0.001 |
| Intermediate stage | 1.66 (1.45-1.91) | 1.50 (1.35-1.65) | ||
| Frail | 2.61 (2.19-3.12) | 1.96 (1.72-2.23) | ||
| Multivariable modelb | ||||
| Robust | 1.00 (referent) | <0.001 | 1.00 (referent) | <0.001 |
| Intermediate stage | 1.50 (1.31-1.72) | 1.41 (1.28-1.56) | ||
| Frail | 1.91 (1.59-2.31) | 1.55 (1.36-1.78) | ||
| Multivariable model + functional limitationsc | ||||
| Robust | 1.00 (referent) | <0.001 | 1.00 (referent) | <0.001 |
| Intermediate stage | 1.35 (1.18-1.55) | 1.35 (1.22-1.49) | ||
| Frail | 1.32 (1.07-1.63) | 1.32 (1.13-1.53) | ||
adjusted for age and site
adjusted for age, site, multimorbidity burden, and cognitive function
functional limitations defined by number of IADL impairments
Findings for outpatient health care costs were similar to those for total health care costs.
Association of the Frailty Phenotype with Hospitalization and Stay in Skilled Nursing Facility
After consideration of traditional indicators including multimorbidity and functional limitations, frail and intermediate stage women had higher odds of subsequent hospitalization, but 95% CIs included null effects (OR 1.41 [95% CI 0.97-2.06] among frail vs. robust women and 1.27 [95% CI 0.96-1.67] among intermediate stage vs. robust women). However, associations of frail and intermediate stage phenotypes with a higher odds of subsequent SNF stay remained strong in magnitude and significant (OR 2.44 [95% CI 1.12-5.34] among frail vs. robust women and 2.24 [95% CI 1.16-4.34] among intermediate stage vs. robust women). In comparison, the odds of a subsequent SNF stay in the fully adjusted model was similar among women with mild vs. no multimorbidity (OR 1.15 [95% CI 0.72-1.86]) and 1.8-fold higher (OR 1.78 [95% CI 1.09-2.90] among women with at least moderate vs. no multimorbidity. Each additional IADL impairment in the fully adjusted model was associated with a 1.2-fold higher odds (OR 1.24 [95% CI 1.09-1.42]) of subsequent SNF stay.
Associations of Individual Frailty Components with Total Health Care Costs
Mean and median total health care costs were higher among women with a given frailty component compared to that among women without that specific component (Supplementary Table 2). Shrinking (CR 1.17 [95% CI 1.01-1.35]), poor energy (CR 1.39 [95% CI 1.24-1.56]), slowness (CR 1.37 [95% CI 1.18-1.59]) and low physical activity (CR 1.44 [95% CI 1.25-1.66]) were each associated with higher mean total costs in models accounting for multimorbidity and cognition (Table 4). The association of weakness with total costs was not statistically significant (CR 1.12 [95% CI 0.98-1.30]). After further accounting for functional limitations, independent associations of poor energy (CR 1.19 [95% CI 1.06-1.34]) and low physical activity (CR 1.16 [95% CI 1.00-1.35]) with higher total costs remained.
Table 4.
Association of Individual Frailty Components with Mean Total Health Care Costs
| Cost ratio (95% CI) | |||
|---|---|---|---|
| Frailty component | Base modela | Multivariable modelb | Multivariable model + functional limitationsc |
| Shrinking | |||
| Present (n=458) | 1.38 (1.20-1.59) | 1.17 (1.01-1.35) | 1.12 (0.97-1.29) |
| Absent | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Weakness | |||
| Present (n=484) | 1.28 (1.11-1.47) | 1.12 (0.98-1.30) | 1.04 (0.90-1.19) |
| Absent | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Poor energy | |||
| Present (n=1163) | 1.56 (1.39-1.74) | 1.39 (1.24-1.56) | 1.19 (1.06-1.34) |
| Absent | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Slowness | |||
| Present (n=476) | 1.72 (1.49-1.98) | 1.37 (1.18-1.59) | 1.00 (0.85-1.18) |
| Absent | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Low physical activity | |||
| Present (n=504) | 1.73 (1.51-1.99) | 1.44 (1.25-1.66) | 1.16 (1.00-1.35) |
| Absent | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
adjusted for age and site
adjusted for age, site, multimorbidity burden, and cognitive function
functional limitations defined by number of IADL impairments
DISCUSSION
In this cohort of older community-dwelling women, greater degree of frailty was associated with higher subsequent total direct health care costs. Importantly, nearly 1 in 5 frail women (and 1 in 10 intermediate stage women) compared with 1 in 25 robust women were among the costliest 10% of individuals. Associations of the frailty phenotype with subsequent total costs and measures of health care burden across inpatient, post-acute care and outpatient sectors remained despite accounting for multimorbidity, functional limitations and cognitive function.
Few previous studies have determined associations of the frailty phenotype with measures of health care burden in older community-dwelling populations. A study of older German adults8 reported that greater degree of frailty (defined by number of CHS frailty components present) was associated with higher total health care costs even after accounting for multimorbidity. However, this study relied on participant’s recall of past health care use to derive cost estimates and did not consider the impact of self-reported function on the association. In addition, a cross-sectional survey in 1284 French adults aged 65 years and older (that made several adaptions to the CHS phenotype based on questionnaire contents) reported that pre-frailty and frailty had an incremental effect on ambulatory health care expenditures even after adjustment for selected self-reported medical conditions and functional limitations.25 In contrast, our study utilized a prospective design, analyzed total health care costs and measures of costs or utilization in inpatient, post-acute and outpatient health care sectors, included a comprehensive assessment of prevalent multimorbidity and defined the frailty phenotype using objective measures of weakness (grip strength) and slowness (gait speed).
Similarly, most investigations examining the association of the frailty phenotype with hospitalization in adults not selected on the basis of disease status are limited by a retrospective design and incomplete adjustment for confounding. Among community-dwelling adults enrolled in CHS4 and National Health and Aging Trends (NHATS)10, the prevalence of self-reported hospitalization in the past year was higher with greater degree of frailty. A survey of European adults age ≥50 years26 found that intermediate stage and frail individuals were more likely to report past hospitalization. While greater frailty status may increase the risk of hospitalization, temporality of the association in these retrospective studies is uncertain because admission to an acute care hospital may also result in the development or progression of frailty. A longitudinal study of older community-dwelling adults residing in Boston27 found that intermediate stage and frail individuals were more likely to report overnight hospitalization and emergency department visits during an average follow-up period of 10 months despite adjustment for demographic factors and cardiovascular risk factors. Our prospective study strengthens earlier findings with its longitudinal assessment of frailty and subsequent claims-based total health care costs and utilization and evaluation of these associations after accounting for comprehensively measured multimorbidity and functional limitations.
Individual frailty components of shrinking, poor energy, slowness and low physical activity level were each associated with higher subsequent total health care costs in our study even after adjustment for multimorbidity. After further consideration of functional limitations, associations of poor energy and low physical activity with health care expenditures remained. These findings are in general agreement with those of previous studies.8;28-31 However, prior investigations have incompletely assessed whether consideration of multimorbidity and self-reported function alter these associations.
Our findings indicate that the frailty phenotype is a risk factor for high subsequent total health care costs above and beyond that explained by burden of chronic medical conditions. Thus, assessment of the phenotype or selected individual components such as poor energy or slow gait may more accurately identify older community-dwelling individuals at higher risk of subsequent health care use, either due to frailty itself or associated functional limitations. High risk individuals would be candidates for targeted treatments aimed at reducing subsequent health care burden including physical therapy, exercise programs, nutritional interventions or enhanced social support services designed to ameliorate functional limitations.32 In addition, in the event of a hospitalization, knowledge of the frailty phenotype or selected components previously assessed in the outpatient setting might improve decision-making among hospital-based clinicians regarding selection of patients for discharge to a post-acute care facility.33 Further research regarding the feasibility and cost/benefit of inclusion of assessment of the frailty phenotype or specific components in the outpatient clinical practice setting is needed.
This study has several strengths including comprehensive participant characteristics available in cohort study data; linkage of participants to their Medicare claims to determine co-existing medical conditions and quantify subsequent health care expenditures and utilization; and consideration of traditional predictors of health care costs. However, this study has limitations. The cohort was comprised of relatively well-functioning community-dwelling older white women. Results may not be generalizable to men, other racial/ethnic groups or those with severe functional limitations such as nursing home residents. However, community-dwelling older adults without overt disability may be most amenable to interventions designed to lower subsequent health care burden. Future studies are needed to replicate our findings in other patient populations. Cost and utilization data were limited to participants enrolled in FFS plans. However, characteristics of participants who were excluded based on not participating in FFS were similar to those included. Furthermore, evidence from the recent decade34 suggests that Medicare FFS enrollees and enrollees in Medicare Advantage (MA) have similar health care expenditures. Our analyses expressed the frailty phenotype using criteria similar to those developed using CHS data, but other instruments have been developed to operationalize the construct of frailty. In particular, additional research is needed to standardize and cross-validate frailty instruments and determine whether results are consistent when the frailty phenotype is assessed using simpler instruments35 suitable for use in the busy clinical practice setting.
In conclusion, the frailty phenotype was an independent determinant of subsequent health care expenditures and utilization, even after accounting for traditional indicators including multimorbidity and functional limitations. These results suggest that assessment of the frailty phenotype or selected components may improve characterization of older community-dwelling adults likely to require more costly, intensive care to better facilitate targeting of interventions designed to reduce future health care burden.
Supplementary Material
Supplementary Figure 1. Participant Flow.
Supplementary Table 1. Characteristics of 5,043 Women at Year 10 Examination by Enrollment Status.
Supplementary Table 2. Mean (SD) and Median (IQR) Total Costs for Women with and without Each Frailty Component.
Table 3.
Associations of Frailty Phenotype with Odds of Hospitalization and SNF Stays
| ≥1 Acute hospital stay | ≥1 SNF stay | |||
|---|---|---|---|---|
| Frailty phenotype | Odds ratio (95% CI) | P | Odds ratio (95% CI) | P |
| Base modela | ||||
| Robust | 1.00 (referent) | <0.001 | 1.00 (referent) | <0.001 |
| Intermediate stage | 1.57 (1.20-2.05) | 2.89 (1.51-5.52) | ||
| Frail | 2.77 (2.02-3.79) | 5.23 (2.62-10.44) | ||
| Multivariable modelb | ||||
| Robust | 1.00 (referent) | <0.001 | 1.00 (referent) | 0.001 |
| Intermediate stage | 1.43 (1.09-1.87) | 2.57 (1.34-4.93) | ||
| Frail | 2.05 (1.47-2.87) | 3.85 (1.88-7.88) | ||
| Multivariable model + functional limitationsc | ||||
| Robust | 1.00 (referent) | 0.16 | 1.00 (referent) | 0.05 |
| Intermediate stage | 1.27 (0.96-1.67) | 2.24 (1.16-4.34) | ||
| Frail | 1.41 (0.97-2.06) | 2.44 (1.12-5.34) | ||
Note: SNF, skilled nursing facility
adjusted for age and site
adjusted for age, site, multimorbidity burden, and cognitive function
functional limitations defined by number of IADL impairments
Impact Statement.
We certify that this work is novel and expands upon findings of previously published cross-sectional studies among community-dwelling adults examining the association of the frailty phenotype with health care costs or hospitalization.1-3 The potential impact of this research on clinical care or health policy includes the following: Assessment of the frailty phenotype or selected individual components such as poor energy or slow gait may more accurately identify older community-dwelling individuals at higher risk of costly and intense health care and improve identification of candidates for targeted treatments including physical therapy, exercise programs, nutritional interventions or enhanced social support services aimed at reducing subsequent health care burden.
- 1.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 2.Bock JO, Konig HH, Brenner H, et al. Associations of frailty with health care costs - results of the ESTHER cohort study. BMC Health Serv Res. 2016;16(1):128. doi: 10.1186/s12913-016-1360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70(11):1427–1434. doi: 10.1093/gerona/glv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Acknowledgments
Conflict of Interest
|
| ||||||
| Financial/Personal Conflicts | KE Ensrud | AM Kats | JT Schousboe | BC Taylor | PM Cawthon | TA Hillier |
|
| ||||||
| Employment or Affiliation | NO | NO | NO | NO | NO | NO |
|
| ||||||
| Grants/Funds | YES | NO | NO | NO | NO | YES |
|
| ||||||
| Honoraria | NO | NO | NO | NO | NO | NO |
|
| ||||||
| Speaker Forum | NO | NO | NO | NO | NO | NO |
|
| ||||||
| Consultant | NO | NO | NO | NO | NO | NO |
|
| ||||||
| Stocks | NO | NO | NO | NO | NO | NO |
|
| ||||||
| Royalties | NO | NO | NO | NO | NO | NO |
|
| ||||||
| Expert Testimony | NO | NO | NO | NO | NO | NO |
|
| ||||||
| Board Member | NO | NO | NO | NO | NO | NO |
| Patents | NO | NO | NO | NO | NO | NO |
|
| ||||||
| Personal Relationship | NO | NO | NO | NO | NO | NO |
|
| ||||||
Drs. Ensrud and Hillier have received grant support from the NIH (and supporting agencies) grant as listed under Funding Sources on the title page.
|
| |||
| K Yaffe | SR Cummings | JA Cauley | L Langsetmo |
|
| |||
| NO | NO | NO | NO |
|
| |||
| NO | YES | YES | NO |
|
| |||
| NO | NO | NO | NO |
|
| |||
| NO | NO | NO | NO |
|
| |||
| NO | NO | NO | NO |
|
| |||
| NO | NO | NO | NO |
|
| |||
| NO | NO | NO | NO |
|
| |||
| NO | NO | NO | NO |
|
| |||
| NO | NO | NO | NO |
|
| |||
| NO | NO | NO | NO |
|
| |||
| NO | NO | NO | NO |
|
| |||
Drs. Cummings and Cauley have received grant support from the NIH (and supporting agencies) grant as listed under Funding Sources on the title page.
Sponsor’s Role: The funding agencies had no direct role in the conduct of the study; the collection, management, analyses and interpretation of the data; or preparation or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the U.S. Department of Veterans Affairs or the United States government.
Source of Funding: The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. The National Institute on Aging (NIA) provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, and R01 AG027576. This manuscript is the result of work supported with resources and use of facilities of the Minneapolis VA Health Care System. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Footnotes
Author Contributions
Kristine E. Ensrud, MD, MPH – study concept and design, acquisition of subjects and data, interpretation of data, and preparation of manuscript
Allyson M. Kats, MS – interpretation of data, critical review of manuscript
John T. Schousboe, MD, PhD – interpretation of data, critical review of manuscript
Brent C. Taylor, PhD – interpretation of data, critical review of manuscript
Peggy M. Cawthon, PhD – interpretation of data, critical review of manuscript
Teresa A. Hillier, PhD – study concept and design, acquisition of subjects and data, interpretation of data, critical review of manuscript
Kristine Yaffe, MD – interpretation of data, critical review of manuscript
Steven R. Cummings, MD – study concept and design, acquisition of subjects and data, interpretation of data, critical review of manuscript
Jane A. Cauley, DrPH – study concept and design, acquisition of subjects and data, interpretation of data, critical review of manuscript
Lisa Langsetmo, PhD – interpretation of data, critical review of manuscript
References
- 1.Congressional Budget Office. High-Cost Medicare Beneficiaries. Washington, DC: The Congress of the United States; 2005. www.cbo.gov. Accessed 12-1-2017. [Google Scholar]
- 2.Neuman T, Cubanski J, Huang J, et al. The rising cost of living longer: analysis of Medicare spending by age for beneficiaries in traditional Medicare. www.kff.org/medicare/report/the-rising-cost-of-living-longer-analysis-of-medicare-spending-by-age-for-beneficiaries-in-traditional-medicare/. Updated 1-14-2015. Accessed 12-1-2017.
- 3.Perkins AJ, Kroenke K, Unutzer J, et al. Common comorbidity scales were similar in their ability to predict health care costs and mortality. J Clin Epidemiol. 2004;57:1040–1048. doi: 10.1016/j.jclinepi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 5.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 6.Cawthon PM, Marshall LM, Michael Y, et al. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;55:1216–1223. doi: 10.1111/j.1532-5415.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- 7.Ensrud KE, Ewing SK, Taylor BC, et al. Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2007;62:744–751. doi: 10.1093/gerona/62.7.744. [DOI] [PubMed] [Google Scholar]
- 8.Bock JO, Konig HH, Brenner H, et al. Associations of frailty with health care costs - results of the ESTHER cohort study. BMC Health Serv Res. 2016;16:128. doi: 10.1186/s12913-016-1360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saum KU, Dieffenbach AK, Muller H, et al. Frailty prevalence and 10-year survival in community-dwelling older adults: results from the ESTHER cohort study. Eur J Epidemiol. 2014;29:171–179. doi: 10.1007/s10654-014-9891-6. [DOI] [PubMed] [Google Scholar]
- 10.Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70:1427–1434. doi: 10.1093/gerona/glv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler A, Gallagher D, Gillespie P, et al. Frailty: a costly phenomenon in caring for elders with cognitive impairment. Int J Geriatr Psychiatry. 2016;31:161–168. doi: 10.1002/gps.4306. [DOI] [PubMed] [Google Scholar]
- 12.Robinson TN, Wu DS, Stiegmann GV, et al. Frailty predicts increased hospital and six-month healthcare cost following colorectal surgery in older adults. Am J Surg. 2011;202:511–514. doi: 10.1016/j.amjsurg.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummings SR, Black DM, Nevitt MC, et al. Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA. 1990;263:665–668. [PubMed] [Google Scholar]
- 14.Schousboe JT, Paudel ML, Taylor BC, et al. Magnitude and consequences of misclassification of incident hip fractures in large cohort studies: the Study of Osteoporotic Fractures and Medicare claims data. Osteoporos Int. 2013;24:801–810. doi: 10.1007/s00198-012-2210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schousboe JT, Paudel ML, Taylor BC, et al. Estimation of standardized hospital costs from Medicare claims that reflect resource requirements for care: impact for cohort studies linked to Medicare claims. Health Serv Res. 2014;49:929–949. doi: 10.1111/1475-6773.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregg EW, Cauley JA, Stone K, et al. Relationship of changes in physical activity and mortality among older women. JAMA. 2003;289:2379–2386. doi: 10.1001/jama.289.18.2379. [DOI] [PubMed] [Google Scholar]
- 17.Sheikh JI, Yesavage JA. Geriatric depression scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. [Google Scholar]
- 18.Whooley MA, Kip KE, Cauley JA, et al. Depression, falls, and risk of fracture in older women. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159:484–490. doi: 10.1001/archinte.159.5.484. [DOI] [PubMed] [Google Scholar]
- 19.Ensrud KE, Nevitt MC, Yunis C, et al. Correlates of impaired function in older women. J Am Geriatr Soc. 1994;42:481–489. doi: 10.1111/j.1532-5415.1994.tb04968.x. [DOI] [PubMed] [Google Scholar]
- 20.Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50:1109–1118. doi: 10.1097/MLR.0b013e31825f64d0. [DOI] [PubMed] [Google Scholar]
- 21.Schousboe JT, Paudel ML, Taylor BC, et al. Pre-fracture individual characteristics associated with high total health care costs after hip fracture. Osteoporos Int. 2017;28:889–899. doi: 10.1007/s00198-016-3803-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schousboe JT, Paudel ML, Taylor BC, et al. Estimating true resource costs of outpatient care for Medicare beneficiaries: standardized costs versus Medicare payments and charges. Health Serv Res. 2016;51:205–219. doi: 10.1111/1475-6773.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20:461–494. doi: 10.1016/s0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 24.Pregibon D. Goodness of link tests for generalized linear models. Applied Statistics. 1980;29:15–24. [Google Scholar]
- 25.Sirven N, Rapp T. The cost of frailty in France. Eur J Health Econ. 2017;18:243–253. doi: 10.1007/s10198-016-0772-7. [DOI] [PubMed] [Google Scholar]
- 26.Ilinca S, Calciolari S. The patterns of health care utilization by elderly Europeans: frailty and its implications for health systems. Health Serv Res. 2015;50:305–320. doi: 10.1111/1475-6773.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiely DK, Cupples LA, Lipsitz LA. Validation and comparison of two frailty indexes: The MOBILIZE Boston Study. J Am Geriatr Soc. 2009;57:1532–1539. doi: 10.1111/j.1532-5415.2009.02394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prina AM, Huisman M, Yeap BB, et al. Hospital costs associated with depression in a cohort of older men living in Western Australia. Gen Hosp Psychiatry. 2014;36:33–37. doi: 10.1016/j.genhosppsych.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Unutzer J, Patrick DL, Simon G, et al. Depressive symptoms and the cost of health services in HMO patients aged 65 years and older. A 4-year prospective study. JAMA. 1997;277:1618–1623. doi: 10.1001/jama.1997.03540440052032. [DOI] [PubMed] [Google Scholar]
- 30.Purser JL, Weinberger M, Cohen HJ, et al. Walking speed predicts health status and hospital costs for frail elderly male veterans. J Rehabil Res Dev. 2005;42:535–546. doi: 10.1682/jrrd.2004.07.0087. [DOI] [PubMed] [Google Scholar]
- 31.Aljadhey H. Physical inactivity as a predictor of high prevalence of hypertension and health expenditures in the United States: a cross-sectional study. Trop J Pharm Res. 2012;11:983–990. [Google Scholar]
- 32.Bibas L, Levi M, Bendayan M, et al. Therapeutic interventions for frail elderly patients: part I. Published randomized trials. Prog Cardiovasc Dis. 2014;57:134–143. doi: 10.1016/j.pcad.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Burke RE, Lawrence E, Ladebue A, et al. How hospital clinicians select patients for skilled nursing facilities. J Am Geriatr Soc. 2017 doi: 10.1111/jgs.14954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newhouse JP, Price M, Huang J, et al. Steps to reduce favorable risk selection in medicare advantage largely succeeded, boding well for health insurance exchanges. Health Aff (Millwood) 2012;31:2618–2628. doi: 10.1377/hlthaff.2012.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–389. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Participant Flow.
Supplementary Table 1. Characteristics of 5,043 Women at Year 10 Examination by Enrollment Status.
Supplementary Table 2. Mean (SD) and Median (IQR) Total Costs for Women with and without Each Frailty Component.


