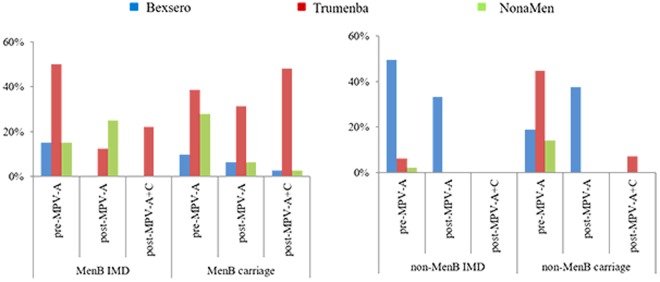
Figure 6.
Prevalence of peptide variants, and potentially immunologically cross-reactive variants, for three serogroup B-substitute vaccines (Bexsero, Trumenba, and NonaMen) among 460 invasive and carriage meningococci from Shanghai, China in the pre-MPV-A, post-MPV-A, and post-MPV-A + C periods. Bexsero and Trumenba are two protein-based serogroup B substitute meningococcal vaccines, which have been licensed in Europe and the USA, while NonaMen is a 9-valent investigational outer membrane vesicle (OMV) vaccine, which has undergone pre-clinical testing. Three periods were defined, pre-MPV-A (1965–1980), post-MPV-A (1981–2008), and post-MPV-A + C (2009–2016), according to the time of two meningococcal polysaccharide vaccines introduced in China (1980 serogroup A, 2008 A and C).