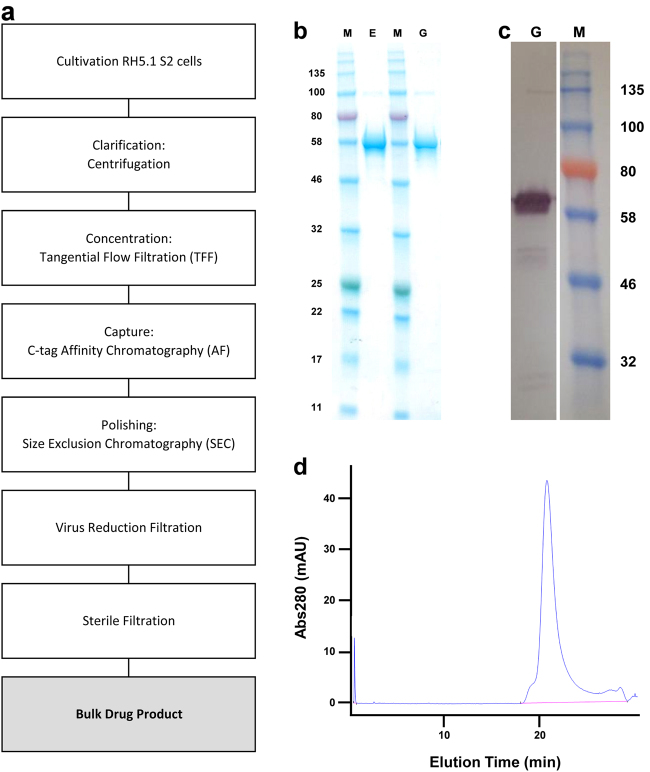
Fig. 2.
Analysis of the purified final RH5.1 drug product. a Overview of RH5.1 protein vaccine cGMP production process. b SDS-PAGE and c western blot (under reducing conditions) of the final RH5.1 drug product produced to cGMP (G) run alongside the comparator engineering batch (E). The western blot used the anti-PfRH5 4BA7 mouse mAb. Within each panel, the gels derive from the same experiment and were processed in parallel. d HPLC-SEC analysis of the final RH5.1 drug product to assess aggregation. M molecular weight markers