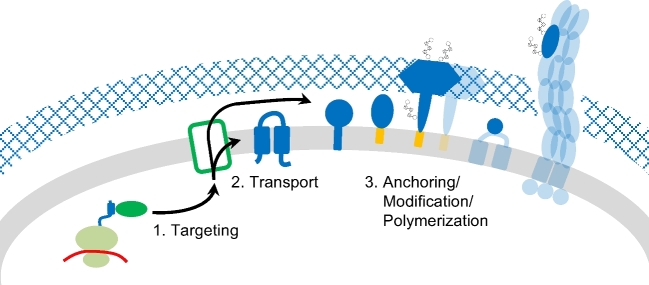
Figure 2.
Schematic overview of common steps in the biosynthesis of archaeal cell surface proteins. During translation, hydrophobic domains of the nascent polypeptide chains are recognized, targeting the ribonucleic protein complex to the membrane (1). The cell surface proteins can be integrated into or transported across the membrane (2), followed by anchoring via transmembrane (TM) domains or covalent linkage to lipid anchors (3). Further post-translational modifications (PTMs) of cell surface proteins include the removal of the signal peptide as well as N- and O-glycosylation. Protein–protein interactions can lead to the binding of soluble proteins at the cell surface or to the polymerization into larger structures such as type IV pili or the S-layer. Proteins involved in distinct pathways associated with these processes (green), the cell surface proteins themselves (blue) as well as their various membrane anchors (orange) are not specified here but discussed in detail in this review.