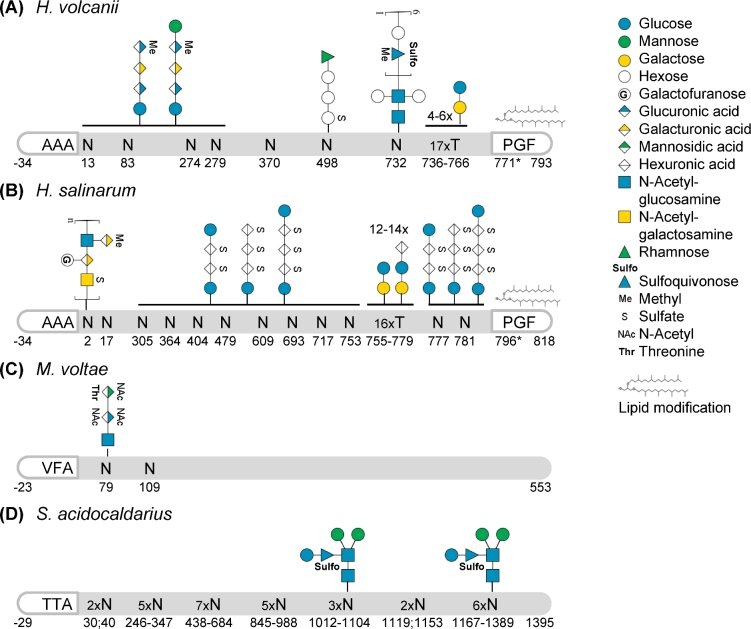
Figure 5.
Post-translational modifications of S-layer glycoproteins. Schematic representations of S-layer glycoproteins from (A)H. volcanii P25062, based on Sumper et al. (1990), Kaminski et al. (2013), Parente et al. (2014), Kandiba et al. (2016), Abdul Halim et al. (2017); (B)H. salinarum B0R8E4, based on Mescher and Strominger (1976), Wieland (1988), Kikuchi, Sagami and Ogura (1999), Jarrell et al. (2014); (C)M. voltae Q50833, based on Voisin et al. (2005); and (D)S. acidocaldarius Q4J6E5, based on Peyfoon et al. (2010). Their signal peptides (last three amino acids before the cleavage site, defined as the peptide bond between positions –1 and +1, are indicated), N- and O-glycosylation as well as lipid modification are highlighted. The position of the PGF motif, conserved for ArtA substrates, is indicated but the processing site has not been resolved yet (*). Glycan compositions are given for confirmed glycosites; solid horizontal lines indicate that all subjacent glycosites were identified with the glycans given above the line. It should be noted that for H. volcanii and S. acidocaldarius shorter glycans of the corresponding N-glycosylation pathways were identified for several N-glycosites and that the extent and type of N-glycosylation can depend on the growth condition. Monosaccharides are depicted according to the Symbol Nomenclature for Glycans (Varki et al.2015). While this is a selection of well-characterized SLG N-glycosylation, a more comprehensive summary can be found in Jarrell et al. (2014).