Abstract
Glucose-stimulated insulin secretion (GSIS) is essential for blood glucose homeostasis and is impaired in type 2 diabetes mellitus. Understanding the regulatory components of GSIS has clinical implications for diabetes treatment. In this study, we found that olfactomedin 4 (OLFM4) is endogenously expressed in pancreatic islet β cells and further investigated its potential roles in glucose homeostasis and the pathogenesis of type 2 diabetes using mouse models. Olfm4-deficient mice showed significantly improved glucose tolerance and significantly increased insulin levels after glucose challenge compared with wild-type (WT) mice. GSIS, mitochondrial ATP production, and mitochondrial respiration were all significantly increased in islets isolated from Olfm4-deficient mice compared with those isolated from WT mice. In a high-fat diet (HFD)–induced diabetic mouse model, the increase in insulin levels after glucose challenge was significantly higher in Olfm4-deficient mice compared with WT mice. The impaired glucose tolerance and insulin resistance in HFD-fed mice were improved by loss of Olfm4. Olfm4 was found to be mainly localized in the mitochondria and interacts with GRIM-19 (a gene associated with retinoid-interferon mortality) in Min6 pancreatic β cells. Collectively, these studies suggest that Olfm4 negatively regulates GSIS. OLFM4 may represent a potential therapeutic target for impaired glucose tolerance and patients with type 2 diabetes.
We investigated olfactomedin 4’s roles in glucose homeostasis and type 2 diabetes. Olfm4-deficient mice showed improved glucose tolerance and increased insulin levels after glucose challenge.
Glucose homeostasis is maintained in large part by pancreatic β cells that secrete insulin in proportion to increasing concentrations of glucose. Pancreatic β cells sense glucose through its metabolism and the resulting increase in ATP, which subsequently stimulates insulin secretion (1). Mitochondria are subcellular organelles where ATP is produced through the oxidative phosphorylation system. Given the importance of oxidative metabolism in β-cell glucose sensing and insulin secretion, it is important to identify the key functional components that regulate mitochondrial activity and ATP production.
Mitochondria complex I [reduced form of nicotinamide adenine dinucleotide (NADH):ubiquinone oxidoreductase] is the largest (at least 45 subunits with a molecular weight of ∼1000 kDa) and most complicated enzyme of five multisubunit complexes (I to V) in the mitochondria that are responsible for oxidative phosphorylation (2). This huge complex has three major functions in the mitochondria. First, it is the major enzyme that oxidizes NADH to NAD+ and thus is responsible for generating the most NAD+ for continued glycolysis and for the function of NAD+-dependent enzymes (3). Second, complex I is the major proton-pumping machine in the mitochondrial inner membrane (4), which drives mitochondrial ATP production. Third, complex I is the major site for cellular production of reactive oxygen species (5). Complex I blockade abolishes glucose-stimulated insulin secretion (GSIS) (6), suggesting complex I activity is absolutely required for GSIS.
Insulin resistance and pancreatic β-cell failure have been identified as being pivotal in the pathogenesis of type 2 diabetes mellitus (T2DM) (7, 8). These observations suggest that functional islet β cells play a pivotal role in T2DM development and restoration of β-cell function and may be one of the key strategies for treatment of T2DM.
Olfactomedin 4 (OLFM4) is a member of the olfactomedin glycoprotein family (9). It is abundantly expressed in bone marrow, neutrophils, gastrointestinal tract, and prostate (9). Olfm4 is a negative regulator of neutrophil defense against a variety of bacteria (10, 11). It is widely used as an intestinal stem cell marker (12) and plays important roles in gastrointestinal inflammation and carcinogenesis (13, 14). OLFM4 has been shown to bind to GRIM-19 (a gene associated with retinoid-interferon mortality), a subunit of the mitochondria complex I (15). Recent genome-wide association studies have linked polymorphisms in OLFM4 with childhood obesity (16). Researchers at Roche have found that OLFM4 serum levels are significantly lower in patients with T2DM and patients with prediabetes than in healthy control individuals [patent application WO 2011064179 A1(17)]. So far, there is no information about OLFM4 in pancreatic islets or its function in glucose and energy homeostasis. In this study, we have identified Olfm4 as a pancreatic β-cell mitochondria protein and show that Olfm4 can act as a modulator of GSIS and glucose tolerance. In a high-fat diet (HFD)–induced diabetic mouse model, Olfm4 deletion leads to improved glucose homeostasis, providing a promising functional benefit.
Materials and Methods
Mice
Male wild-type (WT) and Olfm4-deficient mice at 8 to 10 weeks old on a C57BL/6 background were used in this study (13). For the HFD experiments, at 2 months of age, half of the mice were placed on either a regular chow or 60% fat HFD (D12492; Research Diets, New Brunswick, NJ). The mice remained on regular chow or HFD for 2 months. The animals were maintained in a pathogen-free facility of the National Institutes of Health (NIH; Bethesda, MD). The Animal Care and Use Committee of the National Heart, Lung, and Blood Institute approved the experiments.
Cells, plasmid, and cell culture
Min6 cells (RRID: CVCL_0431) (18) were provided by Dr. Chuxia Deng’s laboratory from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)/NIH. Min6 cells were cultured in DMEM, 10% fetal bovine serum (FBS), and 100 units/mL penicillin and streptomycin in a humidified incubator at 37°C and 5% CO2. His-tag–mouse Olfm4 expression plasmid and empty control vector plasmid were purchased from Origene (Rockville, MD). Olfm4 overexpression stable cell line was established as previously described (19) and maintained in the presence of G418 (500 µg/mL).
Glucose tolerance test and insulin tolerance test
An intraperitoneal glucose tolerance test (GTT) was performed after overnight fasting by intraperitoneal injection of 1 mg/kg glucose. Blood glucose levels were determined with a glucometer (Bayer, Leverkusen, Germany) before (0 minutes) and 10, 15, 30, 60, and 120 minutes after glucose injection. Serum insulin levels during intraperitoneal GTTs were measured with mouse ultrainsulin ELISA kits (Alpco, Salem, NH). Insulin tolerance tests (ITTs) were performed by intraperitoneal injection of insulin (0.75 U/kg) followed by measurement of blood glucose level at the indicated times.
Pancreatic islet isolation
Islets were isolated from WT or Olfm4-deficient mice by the collagenase method (20). Briefly, the mouse pancreas was inflated with Hanks balanced salt solution containing 0.5 mg/mL collagenase type V (Sigma-Aldrich, St. Louis, MO) and kept at 37°C for 20 minutes. The pancreas suspension was overlaid with Ficoll-Paque PLUS (GE Healthcare, Marlborough, MA), and islets were separated from Ficoll/medium interface. Islets were manually collected under a stereomicroscope. Islets were dispersed by incubation with 1 mg/mL trypsin and 30 mg/mL DNase. Islet insulin-producing cells were stained by incubation in the presence of dithizone (100 µg/mL) for 15 minutes before being examined microscopically.
Insulin secretion assay
Islets of similar size were RPMI (10% FBS, 100 units/mL penicillin and streptomycin, and 3 mM glucose) equilibrated for 16 to 24 hours. Islets were suspended in 10 mL Krebs buffer (Sigma-Aldrich) in a 10-cm plate for 10 minutes and transferred to another 10-cm plate with new 10 mL Krebs for 10 minutes. Ten islets were picked and placed in wells of a 96-well plate in 3 mM glucose Krebs buffer or 16.7 mM glucose Krebs buffer. To ensure suspension of secreted insulin, plates were rotated. At 1, 2, 5, 10, and 30 minutes, aliquots of 25 µL were removed from wells. For L-arginine–stimulated (10 mM) or KCl-stimulated (35 mM) insulin secretion assay, aliquots of 25 µL were removed from wells at 20 minutes. After 30 minutes, 1 mL acid-ethanol (ethanol/HCl/H2O, 150:3:147) was added and kept at −20°C for 16 hours to determine total insulin content. All samples were stored at −20°C until being assayed for insulin content (ELISA kit; Alpco).
Intracellular ATP content
Thirty islets cultured and preincubated as described for insulin secretion experiments were incubated for 15 minutes in Krebs/0.1% BSA at 3 mM or 16.7 mM glucose. At the end of the incubation, ATP was extracted from islets and assayed using a luminescent ATP detection assay kit (Abcam, Cambridge, MA).
Measurement of oxygen consumption rate
Oxygen consumption rate (OCR) of mouse islets or Min6 cells was analyzed using the Agilent Seahorse XF analyzer (Agilent Technologies, Santa Clara, CA). On the day of the experiment, islets were transferred to assay media (Agilent Technologies) containing 3 mM glucose and 1% FBS and plated into Agilent Seahorse XP24 Islet Capture Microplates (50 islets per well; Agilent Technologies) according to the manual instruction. The islet plate was then incubated for 60 minutes at 37°C before it was loaded into the XF analyzer. To adjust variation in islet number, OCR of each well was normalized to the initial rates under basal conditions. Min6 cells were cultured in XF96 cell culture microplates (5 × 104 cells per well; Agilent Technologies) overnight. The next day, the medium was changed to assay medium and the OCR analyzed. The OCR was normalized to per-microgram protein. Where appropriate, the cells were treated with glucose (20 mM), the ATP synthase inhibitor oligomycin (1.0 µM), the chemical uncoupler carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP; 1.5 µM), or the electron transport inhibitor antimycin A (1.0 µM). Basal oxygen consumption was assessed before the addition of glucose or any mitochondrial inhibitor.
Histology, immunohistochemistry, and immunofluorescence
Human pancreatic tissue sections were purchased from US Biomax (Rockville, MD). Pancreatic tissues were fixed in 10% buffered formaldehyde and embedded in paraffin. Tissue sections were stained with hematoxylin and eosin. Immunohistochemistry was performed using the Envision Dual Link System-HRP (DAB+) kit from Dako (Carpinteria, CA). The sections were stained with anti-human OLFM4 antibody (RRID: AB_2732892; Sino Biological Inc., Beijing, China) (21), anti-mouse Olfm4 antibody (RRID: AB_2650511; Cell Signaling Technology, Danvers, MA) (22), glucagon antibody (RRID: AB_259852; Sigma-Aldrich) (23), and insulin antibody (RRID: AB_2532448; Thermo Fisher Scientific, Waltham, MA) (24). The pictures were taken with an Olympus BX51 microscope (Olympus, Tokyo, Japan). For immunofluorescence assay, Min6 cells were fixed with methanol, permeabilized with 0.1% Triton, and incubated with anti-mouse Olfm4 antibody (1:100), succinate dehydrogenase A (SDHA) antibody (1:100; RRID: AB_2183449; Santa Cruz Biotechnology, Santa Cruz, CA) (25), or Hsp60 antibody (1:1000; RRID: AB_2264430; Cell Signaling Technology) (26) overnight at 4°C. Secondary Alexa Fluor 488–labeled (1:400; RRID: AB_10374301) (27) or Alexa Fluor 594–labeled antibody (1:400; RRID: AB_2556549) (28) was purchased from Thermo Fisher Scientific, and 4′,6-diamidino-2-phenylindole (Sigma-Aldrich) was used as nucleus counterstaining. The images were taken under a confocal microscope (Zeiss LSM510 META) equipped with 405-, 488-, 594-, and 633-nm lasers and Zen 2009 imaging software using a 63×/1.3 numerical aperture oil-immersion objective (Carl Zeiss, Oberkochen, Germany).
Quantitative RT-PCR, Western blot, and immunoprecipitation
Quantitative RT-PCR, Western blot, and immunoprecipitation assays were performed as previously described (19). Subcellular fraction was performed using a kit purchased from Thermo Fisher Scientific according to the manufacturer’s instructions. GRIM-19 antibody (RRID: AB_2251269) (29) was purchased from Santa Cruz Biotechnology.
Statistical analysis
Two-tailed Student t test was performed to compare results between two groups. One-way ANOVA tests were used for experiments for more than two groups. Differences were considered significant when P was ≤0.05.
Results
Olfactomedin 4 is expressed in pancreatic islet β cells
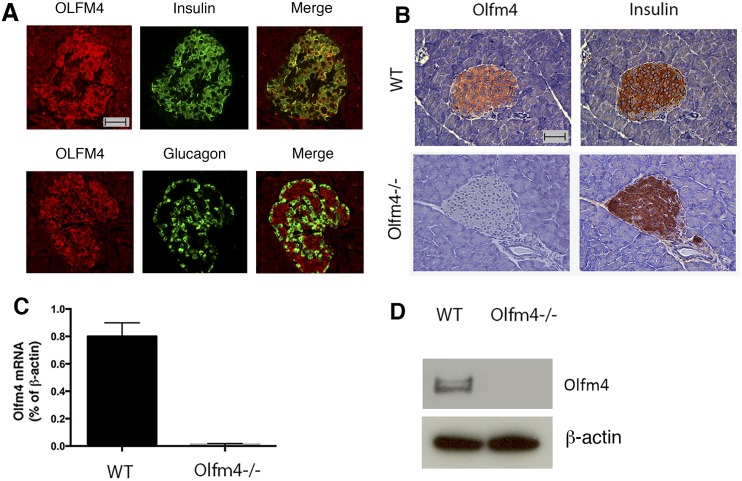
To determine whether olfactomedin 4 is expressed in pancreatic islets, we performed immunofluorescence double staining of OLFM4 with insulin or glucagon in human pancreatic tissues. Olfactomedin 4 expression was observed in the islets and colocalized with insulin in islet β cells, but not with glucagon in α cells (Fig. 1A). In the mouse pancreatic tissues, Olfm4 was also colocalized with insulin in β cells on consecutive tissue sections as demonstrated by immunohistochemistry staining (Fig. 1B). In the islets of Olfm4-deficient mice, Olfm4 expression was absent, whereas insulin expression was not affected (Fig. 1B). Olfm4 mRNA and protein were detected in WT mouse islets but not islets isolated from Olfm4-deficient mice (Fig. 1C and 1D). This report indicates that olfactomedin 4 is endogenously expressed in islet β cells.
Figure 1.
Olfactomedin 4 is expressed in human and mouse pancreatic islet β cells. (A) Immunofluorescence double staining of OLFM4 with insulin or glucagon in human pancreatic tissue sections. Scale bar, 50 µm. (B) Immunohistochemistry staining of Olfm4 and insulin in consecutive pancreatic tissue sections from WT and Olfm4−/− (knockout) mice. Scale bar, 50 µm. (C) Olfm4 mRNA expression in isolated islets from WT and Olfm4−/− mice by quantitative RT-PCR. Expression was normalized to β-actin expression. Data are expressed as mean ± SD (n = 3). (D) Western blot analysis of Olfm4 expression in isolated islets from WT and Olfm4−/− mice. β-Actin was used as a loading control.
Olfm4 deletion improved glucose tolerance and increased GSIS
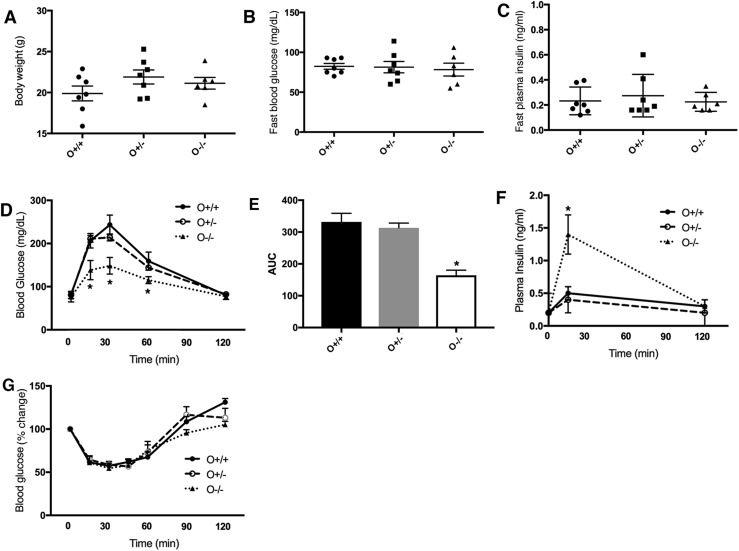
We next explored olfactomedin 4’s potential functions in glucose homeostasis using Olfm4-deficient mice. No difference was observed in weight (Fig. 2A) or blood glucose or insulin levels under fasting (Fig. 2B and 2C) or fed conditions (Supplemental Fig. 1) between WT and Olfm4-deficient mice. However, GTTs showed that glucose tolerance was significantly improved in Olfm4 homozygous-deficient mice compared with WT mice (Fig. 2D). The area under the curve (AUC) in Olfm4 homozygous-deficient mice was significantly lower than those in WT mice and Olfm4 heterozygous-deficient mice (Fig. 2E). Serum insulin levels following glucose challenge were significantly higher in Olfm4 homozygous-deficient mice than in WT mice (Fig. 2F) and coincided with their improved glucose tolerance. Sensitivity to insulin measured by ITTs was not significantly affected by Olfm4 deficiency (Fig. 2G), suggesting that increased insulin secretion by β cells was the primary cause of improved glucose tolerance in Olfm4 homozygous-deficient mice. These results indicate that Olfm4 may play a negative regulatory role for GSIS in islet β cells.
Figure 2.
Olfm4 deletion improves glucose tolerance and increases plasma insulin levels after glucose challenge. (A) Body weight for WT (O+/+) (n = 12), Olfm4 heterozygous- (O+/−) (n = 10), and Olfm4 homozygous-deficient (O−/−) (n = 10) mice. (B) Blood glucose levels for WT and Olfm4-deficient mice after 16-h overnight fasting. (C) Plasma insulin levels for WT and Olfm4-deficient mice after 16-h overnight fasting. (D) Blood glucose levels during GTTs for WT and Olfm4-deficient mice. (E) Area under the curve (AUC) for (D). (F) Plasma insulin levels during GTTs for WT and Olfm4-deficient mice. (G) Blood glucose levels during ITTs for WT and Olfm4-deficient mice. Data are expressed as mean ± SD (n = 3). *P < 0.05 when compared with WT.
Loss of Olfm4 promotes GSIS, ATP production, and mitochondrial respiration activity in islets
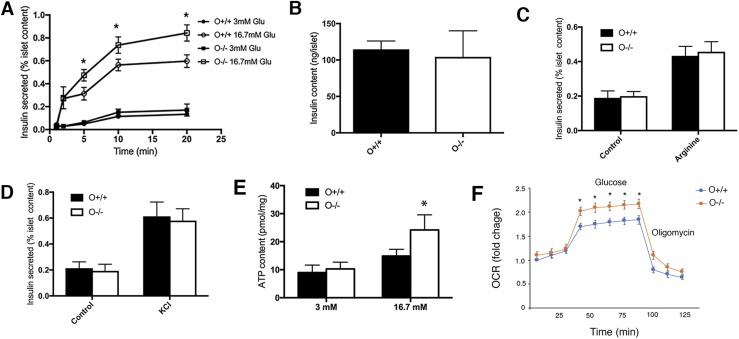
To investigate the mechanisms that may be involved with Olfm4 regulation of GSIS, we analyzed islets isolated from WT and Olfm4-deficient mice. Consistent with the in vivo glucose challenge data (Fig. 2E), the islets from Olfm4 homozygous-deficient mice secreted significantly more insulin than those from WT mice under high-glucose (16.7 mM) stimulation within a short time period (Fig. 3A). No difference was observed for the insulin content in islets isolated from WT and Olfm4-deficient mice and subjected to glucose stimulation (Fig. 3B). We next investigated arginine-stimulated or KCl-stimulated insulin secretion, which were known not to be mediated through metabolism. Both arginine-stimulated and KCl-stimulated insulin secretion in the islets from WT and Olfm4-deficient mice, but no difference for insulin secretion was observed in WT and Olfm4-deficient mice after stimulation (Fig. 3C and 3D), suggesting that Olfm4 was involved in metabolism-mediated insulin secretion.
Figure 3.
Olfm4 deletion increases insulin secretion, ATP production, and mitochondrial respiration in islets isolated from Olfm4-deficient mice. (A) Secreted insulin levels in islets isolated from WT (O+/+) and Olfm4 homozygous-deficient (O−/−) mice in the presence of basal glucose (3 mM) and high glucose (16.7 mM). (B) Insulin content in islets isolated from WT and Olfm4-deficient mice subjected to glucose stimulation. Secreted insulin levels in islets isolated from WT (O+/+) and Olfm4 homozygous-deficient (O−/−) mice in the presence of (C) 10 mM arginine or (D) 35 mM KCl for 20 min. (E) ATP production in islets isolated from WT and Olfm4-deficient mice in the presence of basal glucose (3 mM) and high glucose (16.7 mM). (F) Mitochondria respiration reflected by OCR levels was detected in islets from WT (O+/+) and Olfm4-deficient mice (O−/−) under basal conditions or following the addition of glucose (20 mM) or oligomycin (1 µM). The results presented as fold change compared with basal level. Data are expressed as mean ± SD (n = 3). *P < 0.05 when compared with WT. Glu, glucose.
Because insulin secretion by β cells in response to catabolism of metabolic fuels depends on mitochondrial ATP production (30), we next determined the cellular ATP levels in mouse islets in response to glucose stimulation. The ATP levels in Olfm4-deficient mouse islets were significantly higher than those in WT mice after high-glucose (16.7 mM) stimulation (Fig. 3E). This result further confirms that regulatory effect of Olfm4 in GSIS was mediated by ATP.
We also examined the effect of Olfm4 deletion on the activity of mitochondria respiration activity in mouse islets, because GRIM-19, a binding partner of Olfm4, has been shown to be an essential component of mitochondrial complex I assembly and activity (15) and because mitochondria oxidative phosphorylation is essential for ATP production and insulin secretion (6). In the presence of high-glucose stimulation, the OCR in islets isolated from Olfm4-deficient mice was significantly higher than that in islets isolated from WT mice (Fig. 3F). These results suggest that Olfm4 is a negative regulator for mitochondria respiration and ATP production, both of which are required for GSIS.
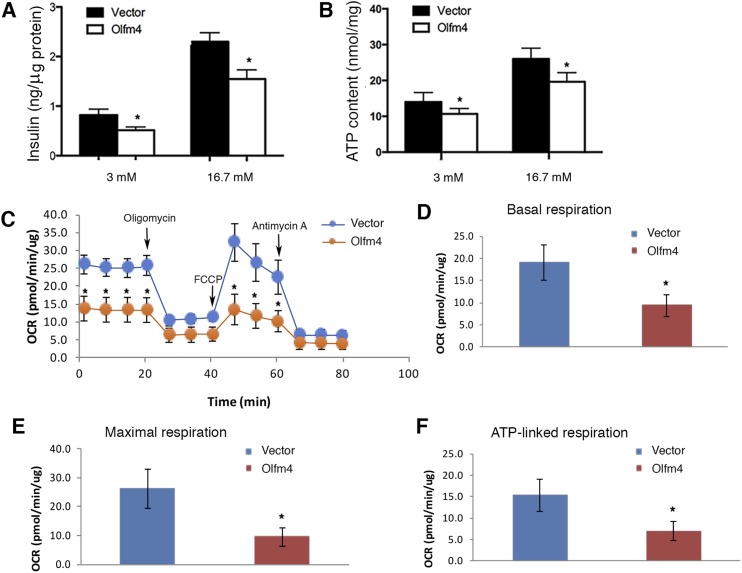
Overexpression of Olfm4 in Min 6 cells decreases insulin secretion, ATP levels, and mitochondrial respiration
Next, we determined whether Olfm4 overexpression would produce the opposite effects of Olfm4 deficiency on insulin secretion, ATP production, and mitochondrial respiration. Using stable pancreatic β Min6 cell lines overexpressing Olfm4, we found that Olfm4 overexpression in Min6 cells led to significantly reduced insulin secretion under both basal (3 mM) and high-glucose (16.7 mM) conditions compared with empty vector control cells (Fig. 4A). Accordingly, ATP production in Olfm4-overexpressing cells were significantly reduced compared with control cells (Fig. 4B). OCR was measured in Olfm4-overexpressing Min6 cells and control cells following treatment with oligomycin, FCCP, and antimycin A (Fig. 4C). The basal respiration, maximal respiration, and ATP-linked respiration in Olfm4-overexpressing Min6 cells was significantly lower than those in control cells (Fig. 4D–4F). Overall, these results suggest that overexpressing Olfm4 in pancreatic β Min6 cells inhibited mitochondrial respiration and ATP production, which in turn decreased both basal insulin levels and GSIS.
Figure 4.
Olfm4 overexpression decreases insulin secretion, ATP production, and mitochondria respiration. (A) Insulin secretion and (B) ATP production from Olfm4-overexpressing or empty vector–transfected pancreatic β Min6 cells in the presence of 3 mM or 16.7 mM glucose. Data are expressed as mean ± SD (n = 3). (C) OCR levels detected in vector or Olfm4-overexpressing Min6 cells under basal conditions or following the addition of oligomycin (1 µM), FCCP (1.5 µM), or antimycin A (1 µM) (n = 5). The rates of (D) basal respiration, (E) maximal respiration, and (F) ATP-linked respiration were quantified by normalization of OCR level to the total protein levels. *P < 0.05 when compared with vector control.
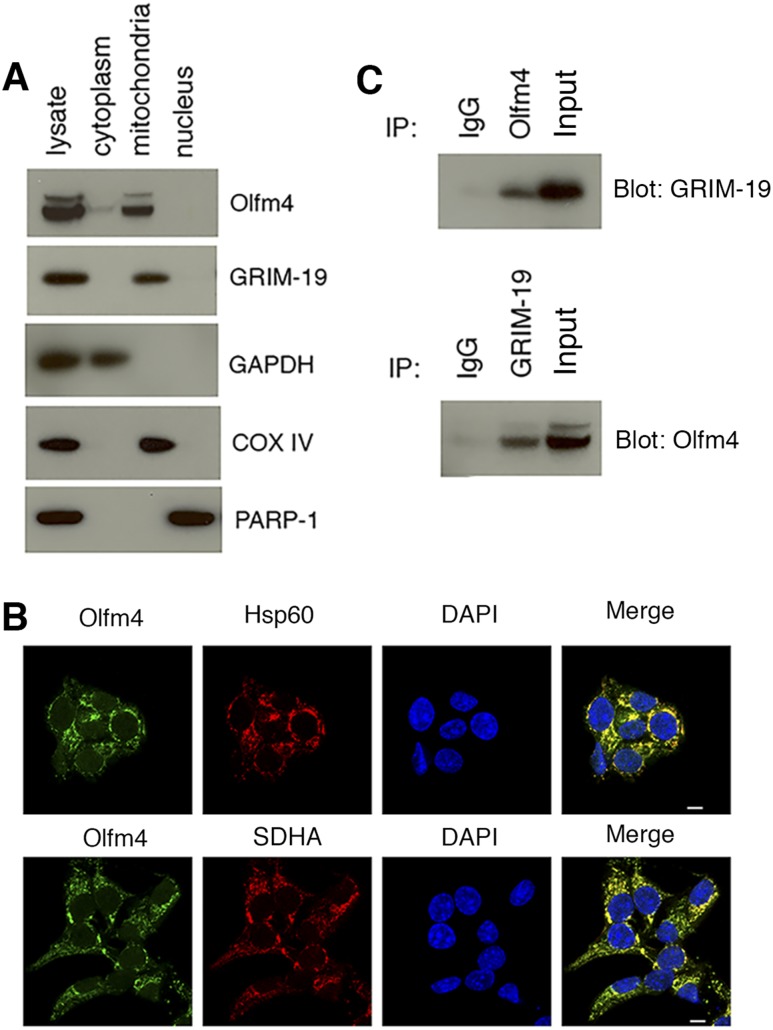
Olfm4 is localized in the mitochondria of pancreatic β cells and interacts with GRIM-19
To explore the molecular basis for these inhibitory effects of Olfm4, we examined the subcellular location of Olfm4 and its interaction with GRIM-19. Western blot analysis of subcellular fractions of Olfm4-overexpressing Min6 cells demonstrated that Olfm4 was primarily localized in the mitochondria (Fig. 5A). Consistent with previous studies (14), GRIM-19 was exclusively expressed in the mitochondria fraction (Fig. 5A). The Olfm4 mitochondria localization was further confirmed using immunofluorescence. Olfm4 staining colocalized with two mitochondria-specific markers, Hsp60 and SDHA (Fig. 5B). Further, Olfm4 coimmunoprecipitated reciprocally with GRIM-19 in Olfm4-overexpressing Min6 cells (Fig. 5C). Given that GRIM-19 is a known subunit of mitochondria complex I, these results suggest that Olfm4 may also be a component of the mitochondria complex I in islet β cells.
Figure 5.
Olfm4 is localized in mitochondria and interacts with Grim-19. (A) Western blot analysis of Olfm4 and GRIM-19 expression in subcellular fractions of Olfm4-overexpressing Min6 cells. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), cytochrome c oxidase (COX) IV, and poly(ADP-ribose) polymerase-1 (PARP-1) were used as subcellular markers for cytoplasm, mitochondria, and nucleus, respectively. (B) Immunofluorescent staining of Olfm4 and Hsp60 or SDHA in Min6 cells. 4′,6-Diamidino-2-phenylindole (DAPI) was used for nuclear staining. Scale bar, 10 μm. (C) Cell lysates from Olfm4-overexpressing Min6 cells were immunoprecipitated (IP) with normal IgG or Olfm4 antibody and subjected to Western blotting with GRIM-19 antibody (top panel). Cell lysates from Olfm4-overexpressing Min6 cells were immunoprecipitated with normal IgG or GRIM-19 antibody and subjected to Western blotting with Olfm4 antibody (bottom panel). The data are representative of three independent experiments.
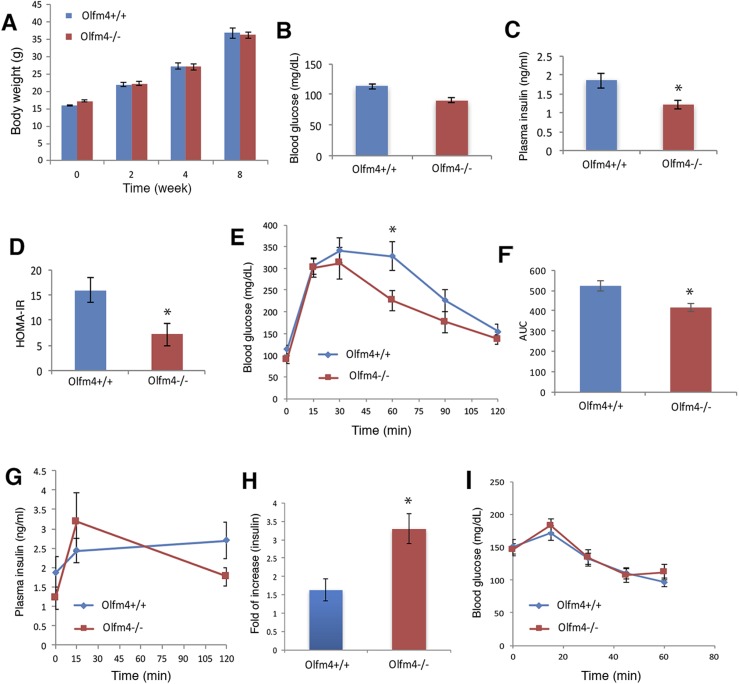
Deletion of Olfm4 increased insulin secretion and improved systemic insulin resistance induced by HFD
Recent studies have shown that an HFD can cause pancreatic β-cell dysfunction and impair insulin secretion in mice (31). These HFD-fed mice displayed insulin resistance and glucose intolerance, which were considered the characteristics of prediabetes (32). To examine the potential involvement of Olfm4 in this diabetes development process, we determined the effect of Olfm4 deletion in an HFD-induced diabetes mouse model. Under an HFD for 2 months, the mice gained weight steadily, but no difference in weight was observed between WT and Olfm4-deficient mice (Fig. 6A). As expected, the fasting glucose and insulin levels in HFD-fed mice (Fig. 6B and 6C) were higher than normal chow-fed mice (Fig. 2B and 2C). Under HFD conditions, the fasting glucose (Fig. 6B) level in Olfm4-deficient mice was lower than those in WT mice, but did not reach statistical significance. However, the fasting insulin level in Olfm4-deficient mice was significantly lower than those in WT mice (Fig. 6C). The homeostasis model assessment of insulin resistance (HOMA-IR) index was significantly lower in HFD-fed Olfm4-deficient mice than in HFD-fed WT mice (Fig. 6D). This finding indicates that loss of Olfm4 could partially restore the systemic insulin resistance induced by HFD. The improved insulin resistance seen in Olfm4-deficient mice under an HFD could be secondary to improved control of diabetes.
Figure 6.
Olfm4 deletion decreases insulin resistance and increases GSIS in HFD-fed mice. Male WT (n = 10) and Olfm4-deficient mice (n = 11) were fed an HFD for 2 months. (A) Body weight of WT and Olfm4-deficient mice. (B) Blood glucose levels in WT and Olfm4-deficient mice after 16-h overnight fasting. (C) Plasma insulin levels in WT and Olfm4-deficient mice after 16-h overnight fasting. (D) HOMA-IR index in WT and Olfm4-deficient mice. (E) Blood glucose levels during GTTs for WT and Olfm4-deficient mice. (F) AUC for (E). (G) Plasma insulin levels during GTTs for WT and Olfm4-deficient mice. (H) Fold increase (over baseline, 0 min) in insulin levels at 15 min in WT and Olfm4-deficient mice. (I) Blood glucose levels during ITTs for WT and Olfm4-deficient mice. Data are expressed as mean ± SD. *P < 0.05 when compared with WT.
We next examined GTTs and GSIS in these HFD-fed mice. Glucose levels at 30, 60, and 90 minutes after glucose challenge were lower in Olfm4-deficient mice than in WT mice with 60 minutes, reaching significant difference (Fig. 6E). The AUC for Olfm4-deficient mice was significantly lower than that for WT mice (Fig. 6F), indicating a significantly improved glucose tolerance in Olfm4-deficient mice under HFD. Examination of insulin levels at 15 minutes after glucose administration during the GTT demonstrated a 1.5-fold insulin-level increase in HFD-fed WT mice and an over threefold insulin increase in HFD-fed Olfm4-deficient mice compared with baseline levels (Fig. 6G). The insulin fold increase was significantly higher in Olfm4-deficient mice than in WT mice (Fig. 6H). These data suggest that the improved glucose tolerance observed in Olfm4-deficient mice is at least partially related to the increased insulin secretion after glucose challenge. This finding is consistent with a protective effect of Olfm4 deficiency on insulin-secretion capacity, even when stressed by an HFD. No difference was observed in insulin sensitivity between WT and Olfm4-deficient mice (Fig. 6I). Collectively, these data suggest that Olfm4 deletion could increase pancreatic β-cell insulin secretion in response to high glucose and improve glucose tolerance under HFD conditions, similar to the findings observed under normal-chow conditions. Moreover, the insulin resistance induced by HFD was significantly improved in Olfm4-deficient mice.
Discussion
GSIS is modulated by several factors, such as nonglucose nutrients, hormones, and neural inputs, and the intracellular network for regulation of insulin secretion is complex and multifactorial (33). Insulin secretion in vivo is finely tuned by a variety of stimulatory and inhibitory signals (33). In this study, our findings in vivo, in isolated islets as well as in β cells, demonstrate a repressive role for Olfm4 in GSIS. This sheds light on Olfm4 metabolic function.
In pancreatic β cells, mitochondria play a central role in coupling glucose metabolism to insulin exocytosis, thereby ensuring strict control of GSIS. Mitochondrial NADH:ubiquinone oxidoreductase (complex I) catalyzes the first step in the electron transport chain, the oxidation of NADH to NAD+ coupled to proton translocation across the inner mitochondrial membrane, and its blockade abolishes GSIS (34). In this study, we demonstrated that Olfm4 is localized in pancreatic β-cell mitochondria and interacts with GRIM-19, which is a known subunit of mitochondrial complex I (35). Unlike GRIM-19, which is essential for assembly and function of mitochondrial complex I (15), loss of Olfm4 under physiological conditions did not affect mitochondria function or ATP production. However, under the high-glucose challenge deletion of Olfm4 enhanced mitochondria respiration, ATP production, and subsequent insulin secretion, suggesting that Olfm4 is a negative regulator of mitochondria activity and ATP production under high-glucose stress conditions.
C57BL/6 mice developed insulin resistance and obesity after feeding with HFD. These model mice displayed insulin resistance and glucose intolerance, which are considered as the characteristics of prediabetes (32). In our study, deletion of Olfm4 improved both glucose intolerance and insulin resistance in HFD mice. The improved glucose tolerance (Fig. 6E and 6F) is at least partially related to the increased insulin secretion after glucose challenge in Olfm4-deleted mouse (Fig. 6G). This is consistent with a protective effect of Olfm4 deficiency on insulin secretion capacity, even when stressed by an HFD. The improved insulin resistance as reflected by lower fasting insulin level (Fig. 6C) and HOMA-IR (Fig. 6D) in Olfm4 deficiency mice under HFD could be secondary to improved control of diabetes. The data that ITT (Fig. 6I) did not change in Olfm4 deficiency mice compared with WT mice suggested that Olfm4 deficiency did not affect peripheral insulin-targeted tissues such as muscles to upload glucose after one shot of insulin injection. This is consistent with the fact that Olfm4 is not expressed in peripheral tissues, including muscles, liver, and adipocytes. Thus, it is less likely that Olfm4 may affect the postinsulin receptor pathway. The beneficial effect of Olfm4 deletion in HFD mice as indicated by lower static fasting glucose and insulin levels suggests that Olfm4 could be a potential therapeutic target of prediabetes or diabetes.
Recently, several genome-wide association analyses have identified and confirmed susceptible loci near the human OLFM4 gene (rs9568856) for childhood obesity (16, 36, 37). So far, there is not any experimental evidence to implicate OLFM4 gene function in obesity. As we previously reported, the body weight of Olfm4-deficient mice did not show any difference with WT mice (14). In this study, we did not observe any effect of Olfm4 loss on body weight and body composition in HFD obesity-related mouse models. These observations suggest that loss of Olfm4 function does not have a direct effect on mouse body weight under normal or some pathological conditions. Whether the single nucleotide polymorphism at rs9568856, which is ∼500 kb from the OLFM4 gene, induces a gain of function remains to be determined.
In summary, we found that Olfm4-deficient mice had increased GSIS and higher ATP levels and mitochondria respiration activity in the islets, establishing that Olfm4 negatively regulates GSIS. Olfm4 deletion has improved diabetes phenotypes in HFD-induced diabetes mouse model. The integration of Olfm4 into the process of insulin secretion in pancreatic β cells makes this protein an attractive novel candidate for drug development in diabetes therapy.
Supplementary Material
Acknowledgments
We thank Dr. Mark Reitman from the NIDDK, NIH, for helpful discussion and careful review of the manuscript.
Financial Support: This study was funded by the Intramural Research Fund of the NIDDK, NIH.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AUC
area under the curve
- FBS
fetal bovine serum
- FCCP
carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone
- GSIS
glucose-stimulated insulin secretion
- GTT
glucose tolerance test
- HFD
high-fat diet
- HOMA-IR
homeostasis model assessment of insulin resistance
- ITT
insulin tolerance test
- NADH
reduced form of nicotinamide adenine dinucleotide
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- NIH
National Institutes of Health
- OCR
oxygen consumption rate
- OLFM4
olfactomedin 4
- SDHA
succinate dehydrogenase A
- T2DM
type 2 diabetes mellitus
- WT
wild-type
References
- 1. Erecińska M, Bryła J, Michalik M, Meglasson MD, Nelson D. Energy metabolism in islets of Langerhans. Biochim Biophys Acta. 1992;1101(3):273–295. [DOI] [PubMed] [Google Scholar]
- 2. Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39(1):359–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaelin WG Jr, McKnight SL. Influence of metabolism on epigenetics and disease. Cell. 2013;153(1):56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hirst J. Mitochondrial complex I. Annu Rev Biochem. 2013;82(1):551–575. [DOI] [PubMed] [Google Scholar]
- 5. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Antinozzi PA, Ishihara H, Newgard CB, Wollheim CB. Mitochondrial metabolism sets the maximal limit of fuel-stimulated insulin secretion in a model pancreatic beta cell: a survey of four fuel secretagogues. J Biol Chem. 2002;277(14):11746–11755. [DOI] [PubMed] [Google Scholar]
- 7. Deng S, Vatamaniuk M, Huang X, Doliba N, Lian MM, Frank A, Velidedeoglu E, Desai NM, Koeberlein B, Wolf B, Barker CF, Naji A, Matschinsky FM, Markmann JF. Structural and functional abnormalities in the islets isolated from type 2 diabetic subjects. Diabetes. 2004;53(3):624–632. [DOI] [PubMed] [Google Scholar]
- 8. Polonsky KS. Dynamics of insulin secretion in obesity and diabetes. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S29–S31. [DOI] [PubMed] [Google Scholar]
- 9. Liu W, Rodgers GP. Olfactomedin 4 expression and functions in innate immunity, inflammation, and cancer. Cancer Metastasis Rev. 2016;35(2):201–212. [DOI] [PubMed] [Google Scholar]
- 10. Liu W, Yan M, Liu Y, McLeish KR, Coleman WG Jr, Rodgers GP. Olfactomedin 4 inhibits cathepsin C-mediated protease activities, thereby modulating neutrophil killing of Staphylococcus aureus and Escherichia coli in mice. J Immunol. 2012;189(5):2460–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu W, Yan M, Sugui JA, Li H, Xu C, Joo J, Kwon-Chung KJ, Coleman WG, Rodgers GP. Olfm4 deletion enhances defense against Staphylococcus aureus in chronic granulomatous disease. J Clin Invest. 2013;123(9):3751–3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van der Flier LG, Haegebarth A, Stange DE, van de Wetering M, Clevers H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology. 2009;137(1):15–17. [DOI] [PubMed] [Google Scholar]
- 13. Liu W, Li H, Hong SH, Piszczek GP, Chen W, Rodgers GP. Olfactomedin 4 deletion induces colon adenocarcinoma in ApcMin/+ mice. Oncogene. 2016;35(40):5237–5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu W, Yan M, Liu Y, Wang R, Li C, Deng C, Singh A, Coleman WG Jr, Rodgers GP. Olfactomedin 4 down-regulates innate immunity against Helicobacter pylori infection. Proc Natl Acad Sci USA. 2010;107(24):11056–11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang G, Lu H, Hao A, Ng DC, Ponniah S, Guo K, Lufei C, Zeng Q, Cao X. GRIM-19, a cell death regulatory protein, is essential for assembly and function of mitochondrial complex I. Mol Cell Biol. 2004;24(19):8447–8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bradfield JP, Taal HR, Timpson NJ, Scherag A, Lecoeur C, Warrington NM, Hypponen E, Holst C, Valcarcel B, Thiering E, Salem RM, Schumacher FR, Cousminer DL, Sleiman PM, Zhao J, Berkowitz RI, Vimaleswaran KS, Jarick I, Pennell CE, Evans DM, St Pourcain B, Berry DJ, Mook-Kanamori DO, Hofman A, Rivadeneira F, Uitterlinden AG, van Duijn CM, van der Valk RJ, de Jongste JC, Postma DS, Boomsma DI, Gauderman WJ, Hassanein MT, Lindgren CM, Mägi R, Boreham CA, Neville CE, Moreno LA, Elliott P, Pouta A, Hartikainen AL, Li M, Raitakari O, Lehtimäki T, Eriksson JG, Palotie A, Dallongeville J, Das S, Deloukas P, McMahon G, Ring SM, Kemp JP, Buxton JL, Blakemore AI, Bustamante M, Guxens M, Hirschhorn JN, Gillman MW, Kreiner-Møller E, Bisgaard H, Gilliland FD, Heinrich J, Wheeler E, Barroso I, O’Rahilly S, Meirhaeghe A, Sørensen TI, Power C, Palmer LJ, Hinney A, Widen E, Farooqi IS, McCarthy MI, Froguel P, Meyre D, Hebebrand J, Jarvelin MR, Jaddoe VW, Smith GD, Hakonarson H, Grant SF; Early Growth Genetics Consortium . A genome-wide association meta-analysis identifies new childhood obesity loci. Nat Genet. 2012;44(5):526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Badi L, Ebeling M, Matile H, Migliorini C, Sabates BJ, Schindler T, Sebokova E, Wang H, inventors. Marker protein for type 2 diabetes. US patent application WO 2011064179 A1. 3 June 2011.
- 18. RRID:CVCL_0431.
- 19. Liu W, Chen L, Zhu J, Rodgers GP. The glycoprotein hGC-1 binds to cadherin and lectins. Exp Cell Res. 2006;312(10):1785–1797. [DOI] [PubMed] [Google Scholar]
- 20. Lernmark A. The preparation of, and studies on, free cell suspensions from mouse pancreatic islets. Diabetologia. 1974;10(5):431–438. [DOI] [PubMed] [Google Scholar]
- 21. RRID:AB_2732892.
- 22. RRID:AB_2650511.
- 23. RRID:AB_259852.
- 24. RRID:AB_2532448.
- 25. RRID:AB_2183449.
- 26. RRID:AB_2264430.
- 27. RRID:AB_10374301.
- 28. RRID:AB_2556549.
- 29. RRID:AB_2251269.
- 30. Detimary P, Dejonghe S, Ling Z, Pipeleers D, Schuit F, Henquin JC. The changes in adenine nucleotides measured in glucose-stimulated rodent islets occur in beta cells but not in alpha cells and are also observed in human islets. J Biol Chem. 1998;273(51):33905–33908. [DOI] [PubMed] [Google Scholar]
- 31. Ehses JA, Meier DT, Wueest S, Rytka J, Boller S, Wielinga PY, Schraenen A, Lemaire K, Debray S, Van Lommel L, Pospisilik JA, Tschopp O, Schultze SM, Malipiero U, Esterbauer H, Ellingsgaard H, Rütti S, Schuit FC, Lutz TA, Böni-Schnetzler M, Konrad D, Donath MY. Toll-like receptor 2-deficient mice are protected from insulin resistance and beta cell dysfunction induced by a high-fat diet. Diabetologia. 2010;53(8):1795–1806. [DOI] [PubMed] [Google Scholar]
- 32. Gao Z, Zhang X, Zuberi A, Hwang D, Quon MJ, Lefevre M, Ye J. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Mol Endocrinol. 2004;18(8):2024–2034. [DOI] [PubMed] [Google Scholar]
- 33. Komatsu M, Takei M, Ishii H, Sato Y. Glucose-stimulated insulin secretion: A newer perspective. J Diabetes Investig. 2013;4(6):511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saraste M. Oxidative phosphorylation at the fin de siècle. Science. 1999;283(5407):1488–1493. [DOI] [PubMed] [Google Scholar]
- 35. Fearnley IM, Carroll J, Shannon RJ, Runswick MJ, Walker JE, Hirst J. GRIM-19, a cell death regulatory gene product, is a subunit of bovine mitochondrial NADH:ubiquinone oxidoreductase (complex I). J Biol Chem. 2001;276(42):38345–38348. [DOI] [PubMed] [Google Scholar]
- 36. Albuquerque D, Nóbrega C, Rodríguez-López R, Manco L. Association study of common polymorphisms in MSRA, TFAP2B, MC4R, NRXN3, PPARGC1A, TMEM18, SEC16B, HOXB5 and OLFM4 genes with obesity-related traits among Portuguese children. J Hum Genet. 2014;59(6):307–313. [DOI] [PubMed] [Google Scholar]
- 37. Felix JF, Bradfield JP, Monnereau C, van der Valk RJ, Stergiakouli E, Chesi A, Gaillard R, Feenstra B, Thiering E, Kreiner-Møller E, Mahajan A, Pitkänen N, Joro R, Cavadino A, Huikari V, Franks S, Groen-Blokhuis MM, Cousminer DL, Marsh JA, Lehtimäki T, Curtin JA, Vioque J, Ahluwalia TS, Myhre R, Price TS, Vilor-Tejedor N, Yengo L, Grarup N, Ntalla I, Ang W, Atalay M, Bisgaard H, Blakemore AI, Bonnefond A, Carstensen L, Eriksson J, Flexeder C, Franke L, Geller F, Geserick M, Hartikainen AL, Haworth CM, Hirschhorn JN, Hofman A, Holm JC, Horikoshi M, Hottenga JJ, Huang J, Kadarmideen HN, Kähönen M, Kiess W, Lakka HM, Lakka TA, Lewin AM, Liang L, Lyytikäinen LP, Ma B, Magnus P, McCormack SE, McMahon G, Mentch FD, Middeldorp CM, Murray CS, Pahkala K, Pers TH, Pfäffle R, Postma DS, Power C, Simpson A, Sengpiel V, Tiesler CM, Torrent M, Uitterlinden AG, van Meurs JB, Vinding R, Waage J, Wardle J, Zeggini E, Zemel BS, Dedoussis GV, Pedersen O, Froguel P, Sunyer J, Plomin R, Jacobsson B, Hansen T, Gonzalez JR, Custovic A, Raitakari OT, Pennell CE, Widén E, Boomsma DI, Koppelman GH, Sebert S, Järvelin MR, Hyppönen E, McCarthy MI, Lindi V, Harri N, Körner A, Bønnelykke K, Heinrich J, Melbye M, Rivadeneira F, Hakonarson H, Ring SM, Smith GD, Sørensen TI, Timpson NJ, Grant SF, Jaddoe VW; Bone Mineral Density in Childhood Study (BMDCS); Early Genetics and Lifecourse Epidemiology (EAGLE) consortium; Early Growth Genetics (EGG) Consortium . Genome-wide association analysis identifies three new susceptibility loci for childhood body mass index. Hum Mol Genet. 2016;25(2):389–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.