Abstract
Cerebral edema is critical to morbidity/mortality in traumatic brain injury (TBI) and is worsened by hypotension. Glibenclamide may reduce cerebral edema by inhibiting sulfonylurea receptor-1 (Sur1); its effect on diffuse cerebral edema exacerbated by hypotension/resuscitation is unknown. We aimed to determine if glibenclamide improves pericontusional and/or diffuse edema in controlled cortical impact (CCI) (5m/sec, 1 mm depth) plus hemorrhagic shock (HS) (35 min), and compare its effects in CCI alone. C57BL/6 mice were divided into five groups (n = 10/group): naïve, CCI+vehicle, CCI+glibenclamide, CCI+HS+vehicle, and CCI+HS+glibenclamide. Intravenous glibenclamide (10 min post-injury) was followed by a subcutaneous infusion for 24 h. Brain edema in injured and contralateral hemispheres was subsequently quantified (wet-dry weight). This protocol brain water (BW) = 80.4% vehicle vs. 78.3% naïve, p < 0.01) but was not reduced by glibenclamide (I%BW = 80.4%). Ipsilateral edema also developed in CCI alone (I%BW = 80.2% vehicle vs. 78.3% naïve, p < 0.01); again unaffected by glibenclamide (I%BW = 80.5%). Contralateral (C) %BW in CCI+HS was increased in vehicle (78.6%) versus naive (78.3%, p = 0.02) but unchanged in CCI (78.3%). At 24 h, glibenclamide treatment in CCI+HS eliminated contralateral cerebral edema (C%BW = 78.3%) with no difference versus naïve. By 72 h, contralateral cerebral edema had resolved (C%BW = 78.5 ± 0.09% vehicle vs. 78.3 ± 0.05% naïve). Glibenclamide decreased 24 h contralateral cerebral edema in CCI+HS. This beneficial effect merits additional exploration in the important setting of TBI with polytrauma, shock, and resuscitation. Contralateral edema did not develop in CCI alone. Surprisingly, 24 h of glibenclamide treatment failed to decrease ipsilateral edema in either model. Interspecies dosing differences versus prior studies may play an important role in these findings. Mechanisms underlying brain edema may differ regionally, with pericontusional/osmolar swelling refractory to glibenclamide but diffuse edema (via Sur1) from combined injury and/or resuscitation responsive to this therapy. TBI phenotype may mandate precision medicine approaches to treat brain edema.
Keywords: : cerebral edema, glibenclamide/glyburide, HS, Sur1, TBI
Introduction
Traumatic brain injury (TBI) affects 1,700,000 people in the United States annually, with a mortality rate of 52,000. The prevalence of resultant disability is estimated at >5,000,000 people.1,2 Secondary insults such as hypotension from polytrauma and hemorrhage are an important contribution to these unfavorable outcomes.3,4 Hypotension is estimated to occur in >25% of severe TBI patients, and can potentially double the mortality rate.4 An analysis of 2061 patients with severe TBI and shock in the Resuscitation Outcomes Consortium trials revealed a mortality rate of 72% in patients with combined injury versus 46% in patients with severe TBI alone.5 A critical factor believed to contribute to unfavorable outcome in these patients is the exacerbation of cerebral edema by secondary hypotension.5 Hypotension is associated with diffuse cerebral edema, distinct from contusional swelling.6 Although much emphasis has been placed on vasogenic edema in TBI at sites of injury/contusion (secondary to traumatic disruption of the blood–brain barrier [BBB]), a growing body of evidence suggests the importance of diffuse cytotoxic edema in this disease, triggered by factors such as global mitochondrial dysfunction, cell depolarization, ionic gradient alteration, and neurotransmitter release contributing to raised intracranial pressure (ICP).7–11 Additionally, the large volumes of resuscitation fluids used to correct hypotension in these patients, although important to restoring an adequate cerebral perfusion pressure (CPP), exacerbate cerebral edema thereby contributing to diffuse swelling and generating a vicious cycle. Given that cerebral edema is one of the most important pathophysiological factors associated with death and unfavorable outcomes in TBI, alternative therapeutic approaches are critically needed.6,12–15
Feinstein and coworkers suggested that resuscitation fluid requirements to restore CPP could be reduced with the use of pressor agents, and although this approach has some merit, it is largely unfeasible in the pre-hospital setting.16 Few studies have been conducted targeting TBI resuscitation in patients with polytrauma. Unfortunately, studies attempting to reduce resuscitation fluid volumes (with either albumin or hypertonic saline) have failed, or even resulted in worse outcomes than patients resuscitated with isotonic crystalloid.17,18 Beyond vasopressors or small volume resuscitation solutions, other therapies targeting cerebral edema are reactionary. Even though osmolar agents, barbiturate coma, hypothermia, or decompressive craniectomy have clinical utility, they are morbid and associated with side effects and/or worsening of hemodynamic status, which may be highly problematic in the setting of polytrauma. Moreover, they are nonspecific and reactive rather than targeted and preventive of cerebral edema. They also have limited use in the pre-hospital setting. A pharmacological strategy given after resuscitation to prevent the progression of cerebral edema rather than treat it in a reactionary manner would be highly desirable to improve outcomes.
One potential strategy in this regard involves targeting a sulfonylurea receptor, Sur1.19 Initially described for its central nervous system (CNS) effects in ischemic stroke, this pathway is now also being studied in TBI.20,21 Sur1 is a transmembrane receptor that obligatorily associates with an adenosine triphosphate (ATP) and calcium-sensitive channel (transient receptor potential cation channel subfamily M member 4 [Trpm4]) and nonselectively conducts monovalent cations.22,23 Injury and depletion of ATP causes upregulation of Sur1 and persistent activation of the Sur1–Trpm4 complex. This results in cell depolarization from sodium influx, causing intracellular edema and eventually cell death.19,24 This pathway has been validated by persistent channel opening and development of edema without ATP depletion by diazoxide24 (which opens the channel), as well as reduction in oncotic cell death with channel blocking by glibenclamide.19 Two major advantages of this pathway over other channels implicated in the process of cerebral edema are that Sur1-Trpm4 is not constitutively expressed in the CNS, but is selectively upregulated by injury, and that it can be inhibited in humans by clinically available United States Food and Drug Administration [FDA]-approved medication for diabetes: glibenclamide, also known as glyburide.
Prior pre-clinical research on Sur1 and TBI20 has focused on inhibiting Sur1 in pericontusional edema and hemorrhage rather than on the diffuse cerebral edema that often causes marked elevation in ICP in TBI patients who have had secondary insults and resuscitation. Additionally, the role of the Sur1 pathway and the impact of agents targeting Sur1 in pre-clinical models of TBI with secondary insults such as hypotension from hemorrhagic shock (HS) have not been examined, despite their potential importance.3,25 To address these issues in evaluating the benefit of glibenclamide as an edema prevention therapy, we used our established model of TBI plus HS in mice.26–28 Also, because Sur1 requires upregulation, the effects on edema may not be apparent in the acute resuscitation period. We hypothesized that mice treated with glibenclamide would have reduced pericontusional and diffuse brain edema at 24 and 72 h after resuscitation. To better understand the contribution of the resuscitation to the development of brain edema after TBI and the impact of glibenclamide, we also studied the impact of glibenclamide therapy in TBI in the presence and absence of secondary HS.
Methods
Injury models
All experiments were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh School of Medicine and Operation Brain Trauma Therapy (approved protocol numbers 14013150, 17019898, and 17091175). Animals were handled in compliance with Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (see online supplementary material at http://www.liebertpub.com). C57/BL6 male mice (Jackson Laboratories, Bar Harbor, ME), 12–15 weeks of age, weighing 25–30 g were used.
Two models were studied: controlled cortical impact (CCI) alone, versus a combined injury model of CCI plus pressure controlled HS. To assess the effect of glibenclamide on cerebral edema at 24 h, mice were randomly divided into five groups (n = 10 per group, Fig. S1) (see online supplementary material at http://www.liebertpub.com): CCI in glibenclamide treatment versus vehicle, CCI+HS in glibenclamide treatment versus vehicle, and naïve. Injury by CCI or CCI+HS was induced in concordance with our standard established models that have been described previously and successfully used in our laboratory for prior investigations.27–29 The level of injury in both CCI and CCI+HS was moderate at 5 m/sec and 1 mm depth to the left parietal cortex. The craniotomies were sealed closed with Koldmount hardener immediately after impact. Procedures were performed during daylight hours in the surgical laboratory.
In the CCI+HS model, HS was induced per protocol by removing 2.3 mL blood/100 g over 15 min, followed by a controlled mean arterial pressure (MAP) of 25–27 mm Hg for 20 min maintained by removal or infusions of citrated autologous blood from the femoral venous catheter in 0.05 mL aliquots. This produced a 35 min period of severe hypotension. Following 35 min of HS, mice entered a “pre-hospital” phase for 90 min in which they were resuscitated with lactated Ringer's (LR) solution for a MAP goal ≥70 mm Hg (initial bolus 20 mL/kg, and additional 10 mL/kg boluses over 5 min as needed to maintain MAP >70 mm Hg). The subsequent “hospital” phase involved reinfusion of the remaining shed blood over 15 min, to mimic clinical care in emergency departments or combat hospitals.
Following glibenclamide treatment versus vehicle (see subsection for dosing), mice were decapitated at 24 h. The brains were removed immediately and the hemispheres were bisected for quantification of brain edema by a technician blinded to treatment. The same protocol was followed to assess the effect of glibenclamide on cerebral edema at 72 h in the combined injury model CCI+HS (n = 9 per group).
For all experiments, mice were anesthetized per our standard protocol with 4% isoflurane with a gas mixture of 2:1 nitrous oxide to oxygen. Isoflurane was reduced to 1–2% after surgical procedures and mice were placed on room air 10 min prior to injury. Isoflurane was maintained at 1% with room air through the 90 min pre-hospital phase and then switched to 100% O2 with 1% isoflurane through the hospital phase.
Glibenclamide infusion and level determination
A stock solution of 2.5 μg/μL glibenclamide was made in 100% dimethyl sulfoxide (DMSO) from which a loading dose solution (20 μg/mL) was made in unbuffered normal saline. These solutions were used for all experiments.
The effect of glibenclamide was assessed at 24 h (n = 10/group/model) and 72 h (n = 9/group/model) using an intravenous (IV) loading dose of glibenclamide (20 μg/kg) that was given 10 min post-CCI, or at the start of the pre-hospital phase in CCI+HS, followed by a continuous subcutaneous (SQ) infusion at 0.4 μg/h (Alzet mini-pump). This treatment regimen was derived as the mouse-equivalent based on rat doses of 10 μg/kg used in prior studies of glibenclamide post-TBI and a body-surface-area-adjusted species conversion.20,30,31 This protocol was used for all mice and continued for 24 h or 72 h after the insult depending on the group being studied. In addition to studies assessing cerebrovascular and systemic hemodynamics after CCI+HS, we also used this protocol in separate mice to quantify glibenclamide levels in blood. Here, the infusion was continued for 4 days.
Glibenclamide levels (n = 5/group) were determined by ultra performance liquid chromatography (UPLC)-mass spectrometer (MS) MS/MS 15 min post-IV load, 1 h post-loading dose plus SQ pump infusion, and at 4 days post-loading dose plus SQ pump infusion to determine steady state levels of the drug. A separate cohort of uninjured mice were used for this determination, because the volume of plasma required by the UPLC MS/MS method were prohibitive in terms of increasing mortality as well as altering our established model. Repeated withdrawal of blood samples in a model of HS would potentially also alter the fluid resuscitation strategy after HS and further influence edema. Blood samples were obtained using cardiac puncture, to avoid potential confounding/dilution by peripheral line draws. Glibenclamide levels were compared with vehicle (n = 3). The UPLC-MS/MS method32 involved liquid-liquid extraction and detection with a triple quadrupole mass spectrometer. Serum (0.2 mL), spiked with glimiperide as internal standard was acidified with hydrochloric acid and extracted with hexanes:methylene chloride (50:50), dried under a gentle stream of nitrogen, and reconstituted in 50 μL of 50:50 acetonitrile:deionized water. Glibenclamide and glimiperide were eluted from a Waters Acquity UPLC BEH C18, 1.7 μm, 2.1 × 150 mm reversed-phase column, isocratically with acetonitrile:water (0.1% formic acid) 50:50. Detection and quantitation were achieved in the positive mode with a Thermo Fisher TSQ Quantum Ultra mass spectrometer interfaced via a heated electrospray ionization (HESI) probe with the Waters UPLC Acquity solvent delivery system. Transitions used for analysis were 494.1 → 368.9 for glibenclamide and 491 → 352 for the internal standard. The calibration curves, obtained from extracting known concentrations of glibenclamide from double-stripped serum, ranged from 0.1 ng/mL (lower limit of quantitation) to 16 ng/mL. All back calculations of calibrators, inter-day and intra-day precision and accuracy, and stability were within acceptable limits. Concurrent glucose levels for all animals at baseline and the abovementioned time points (after HS at 35min, PH at 2h, and HOSP at ∼2.5h) were obtained using a blood gas analyzer (Model ABL-90, Radiometer America, Westlake, OH).
Determination of brain edema
Percent brain water (%BW) was quantified for all mice using the established wet-dry weight technique, which represents a gold standard for its assessment.33 Because perfusion with normal saline alters water levels and provides inaccurate assessment of edema, mice were not perfused with normal saline before brains were harvested for water measurements. Rather, at the completion of the injury described previously, mice were decapitated at each time point (under 5% isoflurane and 50/50 gas mixture of nitrous oxide and oxygen) and the brain was bisected into hemispheres that were immediately weighed; these weights were recorded as wet weights. Hemispheres were subsequently dehydrated for 72 h in an oven at 110°C and re-weighed to record dry weights. %BW was determined by subtracting the dry weight from the wet weight, dividing this number by the wet weight, and multiplying by 100.
Statistical analysis
Glibenclamide levels, glucose concentrations, and ipsilateral and contralateral %BW were reported as means ± standard error. Normality was determined by Q-Q plots (Fig. S2)(see online supplementary material at http://www.liebertpub.com). Differences between %BW in naive, vehicle-, and glibenclamide-treated animals were assessed using one way ANOVA, and between-group comparisons were made using Student's t test. Multiple comparisons during post-hoc analyses were adjusted for using Bonferroni's correction. Physiological parameters were analyzed by repeated measures ANOVA. Outliers were excluded using Dixon's test. Statistical significance was determined by p values <0.05. All statistical tests were conducted using Stata 13.1 (StataCorp, College Station, TX).
Results
Steady state glibenclamide levels do not decrease glucose levels in mice
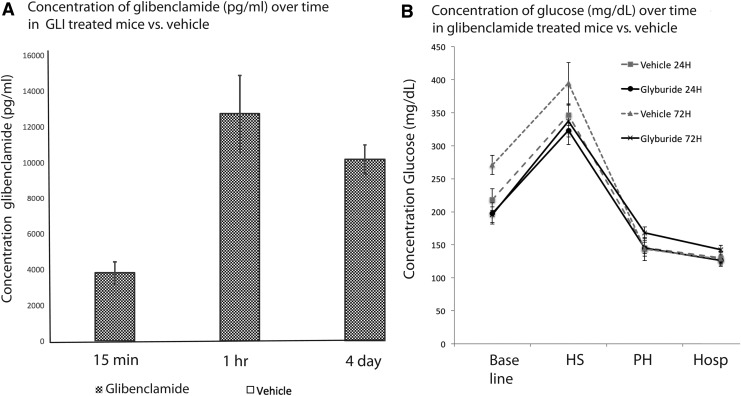
Previous studies in rats suggest that a loading dose of 10 μg/kg intraperitoneal (IP) followed by 200 ng/h of glibenclamide SQ infusion yields a plasma concentration of ∼5000 pg/mL (Simard, unpublished observations) and does not affect serum glucose.19,34,35 Our protocol estimated the effects of an equivalent dose in mice; the 15 min post-IV load levels of glibenclamide were 3809 ± 683 pg/mL. The 1 h post-load level was higher at 12731 ± 2400 pg/mL, and 4 day steady state levels were 10170 ± 911 pg/mL (Fig. 1A). As expected, levels were undetectable in naïve mice. At these levels, serum glucose remained normal, was not significantly different than baseline, and there were no episodes of hypoglycemia in any individual mouse (Fig. 1B).
FIG. 1.
Studies of glibenclamide pharmacokinetics (A) demonstrating immediate post-load levels of glibenclamide (3808 ± 611 pg/mL) and 4 day steady state concentrations (10170 ± 1823 pg/mL). Levels were not detectible in vehicle animals and are therefore not visible in the figure. Therapeutic levels did not influence normoglycemia. (B) Glucose levels were recorded in vehicle- and glibenclamide-treated animals at baseline, and at four subsequent time points: the end of the hemorrhagic shock phase, the end of the pre-hospital phase, the end of the hospital phase, and at 24 h. Glucose levels were not significantly different than in their vehicle counterparts, or than in naïve.
Glibenclamide treatment does not affect ipsilateral edema, but decreases contralateral edema at 24 h after combined injury
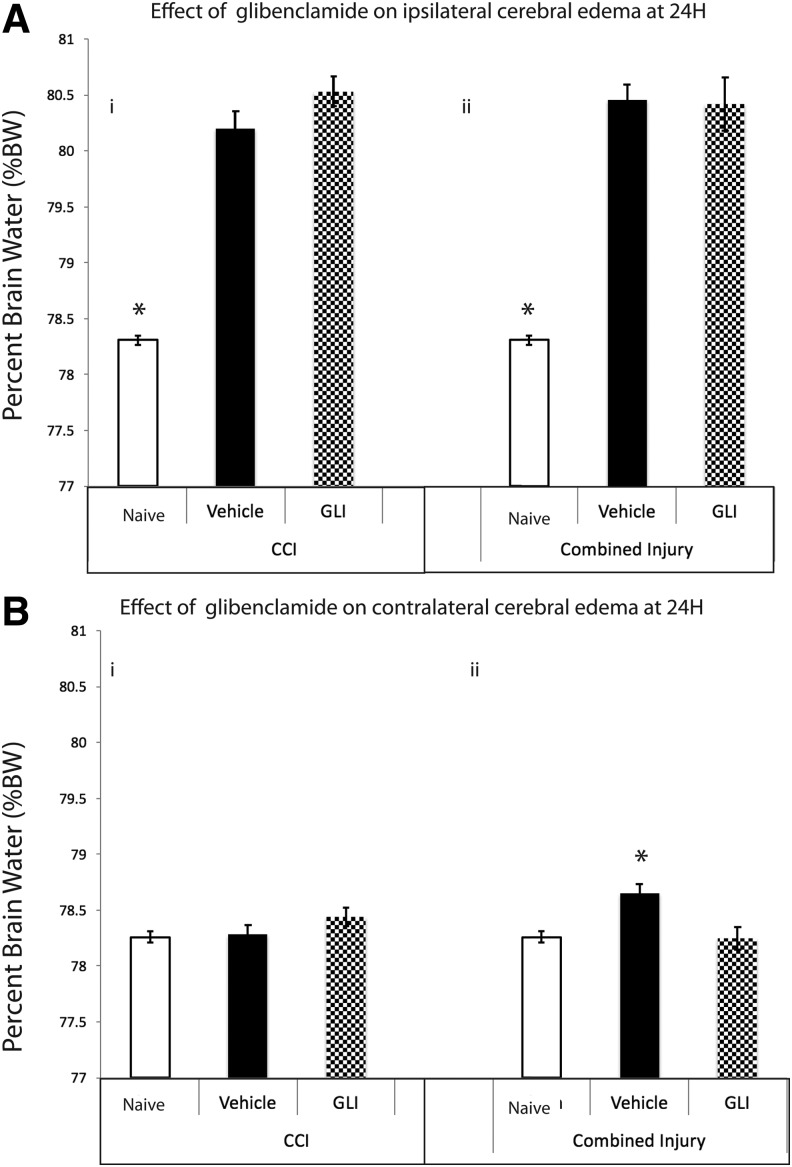
In the contused hemisphere (ipsilateral to TBI), edema was increased at 24 h after CCI+HS (ipsilateral %BW = 80.46 ± 0.14% vehicle [n = 8) vs. 78.31 ± 0.04% naïve [n = 10], p < 0.001) but surprisingly not reduced by glibenclamide (ipsilateral %BW = 80.42 ± 0.24%, p = 1.0, n = 10, power = 1.0 Fig. 2A-i). Edema in the hemisphere ipsilateral to TBI also developed in CCI alone (ipsilateral %BW = 80.20 ± 0.15 vehicle [n = 10] vs. 78.31 ± 0.04% naïve [n = 10] p < 0.001) and again was not attenuated by treatment with glibenclamide (ipsilateral %BW = 80.5 ± 0.13% [n = 10], p = 0.18, power = 1.0, Fig. 2A-ii).
FIG. 2.
(A) At 24 h, %brain water (%BW) in the hemisphere ipsilateral to the contusion was significantly increased in both controlled cortical impact (CCI) (i, vehicle: black bar 80.20 ± 0.15) and CCI+hemorrhagic shock (HS) (ii, vehicle: black bar 80.46 ± 0.14%) versus naïve (white bar, 78.31 ± 0.04%; *p < 0.01). Glibenclamide treatment (shaded gray bar) did not reduce %BW in the hemisphere ipsilateral to contusion in either model. (B) At 24h, %BW in the hemisphere contralateral to the contusion was significantly increased in controlled cortical impact + hemorrhagic shock (CCI+HS) (ii, vehicle: black bar, 78.65 ± 0.10%) versus naive (white bar: 78.24 ± 0.05%, *p = 0.014) but unchanged in CCI alone (i, vehicle: black bar, 78.28 ± 0.08%, p = 1.0). Glibenclamide treatment (shaded gray bar) in CCI+HS (ii) returned %BW to naïve levels (78.25 ± 0.10%, p = 0.011). GLI, glibenclamide.
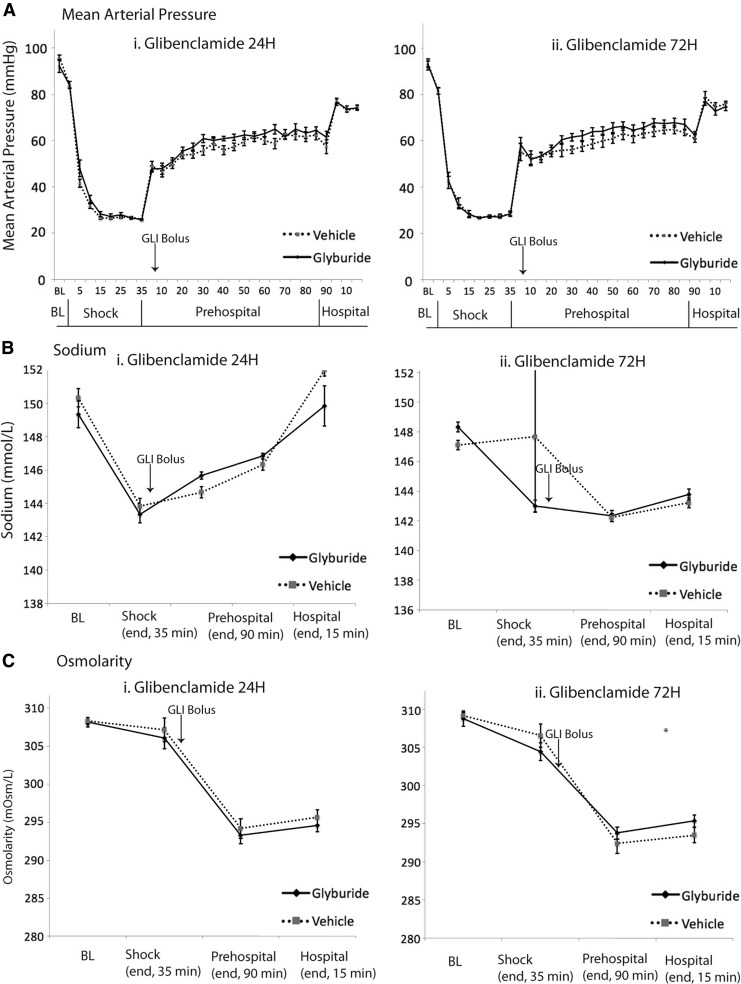
We also detected cerebral edema in the hemisphere contralateral to injury in the CCI+HS model, but not in CCI alone (Fig. 2B). Contralateral %BW in the combined injury of CCI+HS was increased in vehicle (78.65 ± 0.10%, n = 8) vs. naive (78.24 ± 0.05%, n = 10, p = 0.014) but unchanged versus naïve in CCI alone (78.28 ± 0.08%, n = 10, p = 1.0). However, in contrast to what was observed in the hemisphere ipsilateral to injury, at 24 h, glibenclamide treatment after CCI+HS eliminated this low-grade but diffuse brain edema in the contralateral hemisphere versus vehicle (contralateral %BW = 78.25 ± 0.10%, n = 10, p = 0.011; power = 0.904) returning %BW levels to naïve levels (Fig. 2B-ii). It is of note that one of the vehicle-treated mice in CCI+HS died before 24 h. There were no deaths in the glibenclamide-treated group. One CCI+HS vehicle outlier was excluded. There was no difference in physiological parameters that could influence cerebral edema/ICP including MAP, sodium levels, or serum osmolarity between the vehicle and glibenclamide groups (Fig. 3 A-i, B-i, C-i).
FIG. 3.
Continuous recordings of mean arterial pressure (A) were documented during the three phases of the combined injury experiments: hemorrhagic shock (shock, 35 min), pre-hospital phase (90 min), and hospital phase (15 min). Sodium concentration (B) and serum osmolarity (C) were also measured at the following time points: baseline, at the end of each phase of combined injury (“shock” at 35 min, “pre-hospital” at 125 min,“hospital” at 140 min), and at 24 h in the high-dose experiments. These measurements were performed comparing vehicle- with glibenclamide-treated animals for 24 h (A-i, B-i, C-i) and for 72 h (A-ii, B-ii, C-ii).
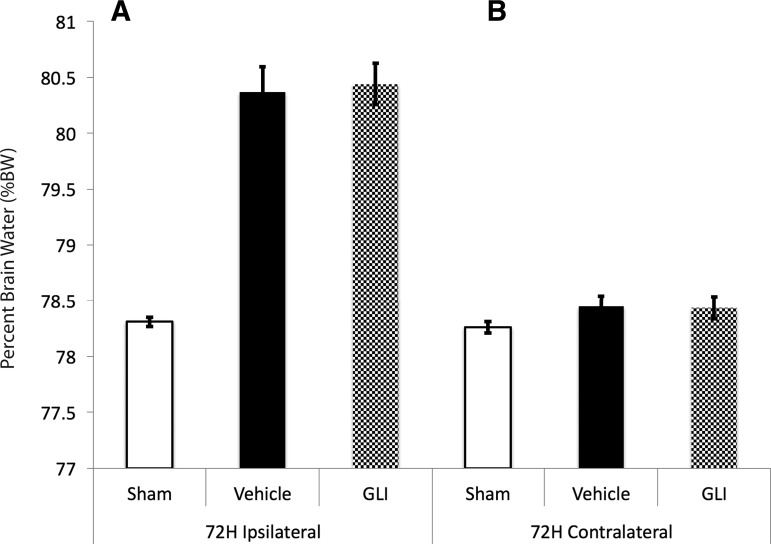
Glibenclamide treatment does not affect 72 h ipsilateral edema in combined injury
Ipsilateral edema remained increased at 72 h in CCI+HS (ipsilateral %BW = 80.37 ± 0.04% vehicle vs. 78.31 ± 0.04% naïve, p < 0.001), but again was not reduced by glibenclamide (p = 1.0, power = 1.0, Fig. 4A). Contralateral edema largely resolved in the combined injury model by 72 h (contralateral %BW = 78.45 ± 0.09% vehicle vs. 78.26 ± 0.05% naïve, p = 0.24, power = 0.46, Fig. 4B). Two animals in both vehicle (n = 9) and glibenclamide (n = 9) treated groups died by 72 h after injury, consistent with the observed level of mortality in our prior reports with this severe combined injury model.21 MAP, serum sodium levels, and serum osmolarity were not different between the treatment and vehicle groups (Fig. 3 A-ii, B-ii, C-ii).
FIG. 4.
At 72 h, (A) in the hemisphere ipsilateral to contusion, %brain water (BW) was increased in vehicle (black bar, 80.37 ± 0.04%) versus naïve (white bar, 78.31 ± 0.04% naïve, p < 0.001). Glibenclamide treatment (shaded gray bar) did not reduce ipsilateral %BW. (B) At this time point, %BW in the hemisphere contralateral to the contusion had resolved (vehicle 78.45 ± 0.09% vs. naïve 78.26 ± 0.05%, p < 0.24).
Discussion
Our study demonstrates the following findings: (1) a combined injury model of TBI (by CCI) and hypotension (by HS) followed by resuscitation with LR generates a significant amount of edema in the contused hemisphere, and also results in diffuse edema in the contralateral hemisphere; (2) in this injury model and treatment regimen, glibenclamide decreases (or prevents generation of) edema in the contralateral hemisphere back to baseline by 24 h; (3) contralateral cerebral edema is largely resolved by 72 h; (4) in mice with isolated CCI, edema is restricted to the hemisphere ipsilateral to the impact: and (5) surprisingly, in both models, our dose of glibenclamide treatment failed to attenuate edema in the contused hemisphere ipsilateral to impact.
These data suggest that combined injury pre-clinical models may provide unique insight relevant to the study of cerebral edema in TBI. They emphasize that cerebral edema in TBI is complex, and that a “one-size-fits-all” approach to developing therapies that do not consider mechanistic differences between types of edema, or tailored timing of treatment may be inadequate. The secondary HS and/or resuscitation after CCI (mimicking scenarios after human TBI, particularly in polytrauma) further adds to the complexity. This second insult leads to the development of a unique low-grade diffuse edema in the contralateral hemisphere. Based on these findings, in the CCI model, and at the injury levels used in our studies, standard-dose glibenclamide treatment appears to represent a better therapy to target this diffuse cerebral edema than contusional swelling. The focus of our study was limited to the effect of glibenclamide on cerebral edema in two different models of TBI. It lays the foundation for important future work beyond the scope of this investigation to evaluate whether this effect translates to improvement in outcomes such as lesion volume, cognition, or motor function in the important setting of TBI complicated by shock, polytrauma, and fluid resuscitation. Our results also lay the foundation for future studies characterizing the optimal timing and duration of therapy as well as the underlying molecular nuances in combined injury models.
Diffuse cerebral edema and secondary insults in TBI
The fact that secondary insults such as hypoxia and hypotension increase morbidity and mortality after TBI has been well established.36 Indeed, hypotension has been cited as the single most important prognostic factor36 and occurs in a significant proportion of TBI patients. The underlying mechanisms explaining this association, however, remain to be determined. The effects of cerebral edema likely play a critical role in this process, as it is also significantly linked to morbidity and mortality in TBI, and is exacerbated by hypotension and resuscitation efforts.6,12–15,27 Some insight into the exacerbation of brain edema by secondary insults was provided by important studies conducted in the laboratory of Marmarou and his co-investigators. Using an impact acceleration weight-drop model of diffuse TBI in rats and diffusion-weighted MRI, Ito and coworkers demonstrated that even with an injury level that alone produced no detectible brain edema, 30 min of secondary hypotension to an MAP of 30 mm Hg plus hypoxemia (PaO2 of 40 mm Hg), produced diffuse edema and substantial intracranial hypertension at 3 h after the combined insult.8 Subsequent studies suggested that the second insult impaired the ability of the brain to restore ion homeostasis, including potassium and sodium.37 They strongly implicated cytotoxic edema as the mechanism underlying the formation of diffuse edema in their model. Our results are consistent with these findings, broaden them from a diffuse injury model to CCI, and demonstrate that although the degree of ipsilateral edema is similar in CCI with or without a secondary hypotensive insult, diffuse edema in the contralateral hemisphere is seen only in the combined injury model of CCI+HS and is absent in CCI alone.
Ipsilateral edema is not responsive to this dose of glibenclamide therapy in mice (20 μg/kg loading dose followed by 0.4 μg/h SQ infusion)
The failure of glibenclamide to attenuate edema in the contused hemisphere in our study, although unexpected, supports the growing body of literature demonstrating that there are multiple mechanisms underpinning different types of cerebral edema generated in TBI and that these mechanisms may require unique and targeted therapies and specific doses/durations of therapy. It is possible, indeed likely, that the Sur1/glibenclamide pathway is one of many upregulated edema-generating mechanisms, and that the effects of its inhibition may therefore be diluted/overwhelmed by other mechanisms. These mechanisms may include a profound osmolar gradient caused by the contusion resulting in edema refractory to Sur1-targeted therapy even though Sur1 upregulation has been demonstrated in the contusion/pericontusion.20,30,38,39 It is also possible that glibenclamide treatment at this dose may influence additional targets other than Sur1 related to cerebral edema and/or neuroprotection.40 The complexity associated with categorization of cerebral edema after TBI was recognized as far back as 1905 when Reichardt coined the term “brain edema” to differentiate underlying cytotoxic “brain swelling” from vasogenic edema caused by vascular engorgement.9 Advances in research have since delineated a number of molecular pathways involved in the pathogenesis of TBIrelated cerebral edema such as aquaporin-4 (AQP4), high-mobility group protein B1 (HMGB1)-toll-like-receptor-4 (TLR4), matrix metalloproteinases (MMP), Na+-K(+)-2Cl(-) cotransporter (NKCC1), and, germane to our investigations, Sur1–Trpm4.21,31,41–52 These mechanisms affect various processes such as cellular volume regulation, oncotic gradients, BBB integrity, and inflammatory responses, culminating in different forms of brain edema that have been described as contusional versus diffuse, or categorized as vasogenic, cytotoxic, or osmotic.9
The ipsilateral edema in the CCI and CCI+HS models may primarily be contusional in nature, generated because of an osmotic potential across the central necrotic tissue (high osmolality) and the peripheral surrounding brain regions.38,39 Katayama and coworkers characterized the spatial and temporal pattern of this edema in animal models by measuring contusion and pericontusional osmolality and water content.38 This was confirmed in patients with apparent diffusion coefficient imaging.39 In our study, ipsilateral edema was not responsive to glibenclamide treatment at either 24 or 72 h, suggesting that the Sur1 pathway (or other ion channels) may be less prominent, or overwhelmed by alternative edema-generating mechanisms in the contusional site. Additionally, glibenclamide treatment may have other targets/an alternate role in the ipsilateral hemisphere/contusion compared with diffuse edema generation. A recent study suggests that glibenclamide attenuates BBB disruption after TBI via a c-Jun N-terminal kinase (JNK)/c-jun signaling pathway in endothelial cells.40
Diffuse cerebral edema after combined injury is responsive to this dose of glibenclamide therapy at 24 h (20 μg/kg loading dose followed by 0.4 μg/h SQ infusion)
Earlier theories of the etiology of edema in TBI being caused by vascular engorgement have now been replaced with evidence that there is usually a key cytotoxic component, particularly to peri-lesional and diffuse edema.8,10,53–57 In addition, some component of vasogenic edema thought to be secondary to BBB compromise may also contribute to diffuse edema.10,58,59 BBB permeability to albumin was not seen in the contralateral hemisphere in CCI alone.60 The Sur1 pathway has been implicated in its contributions to both cytotoxic and vasogenic components of brain edema: association with Trpm4 in neurons causes rapid cell depolarization, influx of sodium, followed by intracellular (i.e., cytotoxic) edema, and eventually oncotic death. The same process in CNS vascular endothelial cells results in degradation of the tight junctions and compromises the BBB, allowing extravasation of proteinaceous fluid (i.e., vasogenic edema).62 Prior studies evaluating the effect of glibenclamide in TBI have used standard models of focal cortical contusion and predominantly focused on the ipsilateral hemisphere, hippocampal injury, and impact on parenchymal hemorrhage.20,21 Although increased Sur1 expression has been detected in the contralateral hemisphere after injury,20 its role particularly as it relates to diffuse edema has been characterized to a lesser extent. This is likely because models of CCI alone, at various injury levels, typically do not produce diffuse brain edema as demonstrated by our study and other reports, when water content in contralateral hemispheres is tested separately from hemispheres ipsilateral to injury.8,31,62–71
Our model of CCI+HS therefore provides a useful opportunity to evaluate the effect of standard-dose glibenclamide on edema, given the diffuse edema generated in the contralateral hemisphere. Remarkably, this edema was virtually eliminated by treatment with glibenclamide at 24 h. Although this suggests an important role of the Sur1 pathway in the development of this type of edema, potential off-target effects of glibenclamide on secondary brain injury and neuroprotection are also a consideration. A molecular examination of the differential contributions of various pathways to diffuse edema and their response to glibenclamide in an edema-generating model with combined injury merits exploration.
Although deceptively small, a 0.5% increase in BW is often clinically significant. For example, after 9 min asphyxial cardiac arrest, an ∼0.5% global increase in BW is seen and associated with significant neuronal death and neurological impairment.72 After TBI, a 0.5% increase in BW in the uninjured brain appears to contribute to elevations in ICP, suggesting clinical relevance: in CCI alone, ICP increases to ∼13–14 mm Hg and is not affected by glibenclamide;31 however, the addition of shock in our model results in an ICP increase to ∼20 mm Hg by the end of the resuscitation.27 This diffuse edema is challenging to treat, and in our model of CCI+HS the only agents to decrease contralateral %BW have been an experimental blood substitute (polynitroxylated pegylated hemoglobin-A27), and glibenclamide as shown in our current report. Agents failing to reduce this seemingly minimal contralateral brain edema in our hands have included an AQP4 blocker, 23.4% NaCl, and whole blood (unpublished data). In Operation Brain Trauma therapy (OBTT) which studies pre-clinical therapies in TBI alone, of the many agents investigated, the second most positive therapy to date is glibenclamide.73 In OBTT, glibenclamide reduced contusion volume and improved motor function specifically in the CCI model; however, consistent with this current report, contusional %BW was not reduced, again suggesting a potential effect of glibenclamide independent of contusional edema.73
An important limitation of our study is that ICP was not recorded. This was for several reasons. Our previously published work on this model has already demonstrated the clinically relevant increases in ICP by the end of the resuscitation.27 Given that the Sur1–Trpm4 channel is not present or at very low levels in the uninjured brain, but requires upregulation,59 it was not determined to be high yield to monitor ICP during the glibenclamide infusion during the acute phase, particularly as invasive monitoring can induce some level of damage and cause confounding effects. Although it would have been interesting to follow ICP and CPP out to the 24 h time point in our studies, maintaining mice anesthetized and monitored for extended periods of time is highly problematic.
Although we have previously characterized many of the consequences of the secondary insult, including worsening of functional outcome and neuropathology in our combined injury model,18, 21 we have not defined whether or not the HS leads to BBB dysfunction in the hemisphere contralateral to injury. Therefore, we cannot definitively say whether the benefit of glibenclamide on diffuse edema in our model results from the effects on cytotoxic or vasogenic edema or both.
Because Sur1 is upregulated, its contribution to diffuse edema and inhibition with glibenclamide may not be maximized in the 24 h period. Recent clinical studies in 28 patients with severe TBI demonstrated a strong correlation between early edema and cerebrospinal fluid (CSF) Sur-1 levels, along with a correlation between resolution of intracranial hypertension and a fall in CSF Sur-1 levels between 48 and 72 h after TBI.74 In our mouse model, diffuse edema in injured mice had resolved by 72 h; %BW in both vehicle- and glibenclamide-treated groups was no different than in naive. Additional studies with time points between 24 and 72 h using either a more severe CCI or a more severe second insult (or both) may provide further insights into the optimal timing and duration of glibenclamide treatment. Also, more highly regional assessments than hemispheric %BW are indicated either with focused tissue sampling or MRI. It is also possible that different doses of glibenclamide may be needed to modulate brain edema in various brain regions in either CCI or our combined injury model, depending on the mechanism of edema formation in any given region.
Glibenclamide in TBI
Our work is unique compared with other pre-clinical studies of glibenclamide in TBI in terms of the species (mouse), the model (combined injury vs. CCI alone), and the outcome (beneficial effects of the standard glibenclamide dose was only seen in relatively mild contralateral cerebral edema but not in significant contusional edema). To our knowledge, with the exception of a recent study by Xu and coworkers,40 pre-clinical studies on the effect of glibenclamide in TBI have primarily been conducted in rat models.20,30,31,59 Although there have been a few mouse-based reports of Sur1 in spinal cord injury (SCI)75 and CNS preconditioning,76 animal studies investigating Sur1 and glibenclamide in acute ischemic stroke, SCI, cardiac arrest, and subarachnoid hemorrhage have mostly been in rats.19,22,23,35,59,77–81 One advantage of using a mouse model is that it facilitates future exploration of this pathway in knockout animals. However, this pathway is less well characterized in mice.
There have been four published pre-clinical studies evaluating glibenclamide specifically in TBI.20,30,31,40 These studies examine CCI alone, and none evaluate glibenclamide in a combined model of CCI plus a secondary insult such as HS. In two of these studies (using 10 μg/kg glibenclamide in rats) reduced progressive secondary hemorrhage and improved behavioral outcomes were noted; however, cerebral edema was not assessed.20,30 Edema was evaluated by Zweckberger and coworkers in a rat model of CCI: 10 μg/kg of glibenclamide treatment did not affect acute ICP but decreased 24 h ipsilateral BW and contusion volume (at 8 h, 24 h, 72 h, and 7 days).31 We cannot rule out the possibility that the variation between these findings and our results are the result of differences in dosing between mice and rats as well as the fact that the therapeutic level achieved by these other studies is unknown/unpublished as distinct from our study with steady state levels of ∼10 ng/mL achieved after a loading dose of 20 μg/kg (calculated murine equivalent of 10 μg/kg in rats) followed by 0.4 μg/h infusion. The most recent study by Xu and coworkers41 also evaluated isolated CCI and brain edema in mice. They reported reduction in ipsilateral %BW (at day 3 post-TBI) and BBB disruption after 10 μg intraperitoneal injection of glibenclamide for 3 days, and implicated the role of an alternative pathway (JNK/c-jun mediated apoptosis). Although these reports confirm our finding of no contralateral edema development in rats or mice after CCI alone, contrary to our results, both reported reduction in ipsilateral BW at 24 h.
There are multiple essential differences between these reports and our study including (1) methodologies to determine brain edema, (2) glibenclamide treatment dose, (3) treatment duration, (4) species (5) injury severity, and (6) timing of the assessments. Although we used body-surface-area-adjusted dose conversions to achieve the murine equivalent of 10 μg/kg in rats, and with this regimen achieved similar post-load glibenclamide levels compared with unpublished observations by Simard and coworkers,35 our steady state levels were ∼10 ng/mL (20 nM). It is unknown how this steady-state level compares with four other studies mentioned previously, because those data were not published. It is of note that the Half maximal effective concentration (EC50) of glibenclamide is 48 nM at a pH of 7.4, which is higher than our level of 20 nM. Glibenclamide potency significantly increases with decreasing pH (that may be encountered in ischemic/damaged tissue); the EC50 of glibenclamide at a pH of 6.8 is 6 nM.82 Therefore, the regional effects of glibenclamide may vary based on the local microenvironment. Other reports of glibenclamide in murine models of non-TBI disease use higher doses (10 μg/mouse) with reduced in vivo neuronal damage, preservation of myelin, preservation of axons, and more numerous/mature oligodendrocytes and reduced in vitro glutamate-induced cell swelling in experimental autoimmune encephalitis.83,84 This, combined with the results from Xu and coworkers, indicates that differences in murine dosing may be an important contributor to the variation in results observed between our study and prior reports.
The varied results of these pre-clinical studies highlight the complexity and heterogeneity of TBI, and, therefore, the importance of studying different doses, treatment durations, models, species, and injuries so that pre-clinical models can more accurately inform clinical studies. Indeed, a recent small randomized controlled trial in moderate-severe TBI (diffuse axonal injury) suggested that glibenclamide may be neuroprotective. The mechanism of neuroprotection is currently unclear, and may include pathways distinct from cerebral edema.85 Although negative murine studies may not be directly predictive of results in humans, they can inform optimal treatment regimens and clinical trial design. Our data suggest that the setting of TBI complicated by HS, polytrauma, and fluid resuscitation merits additional study of the potential utility of glibenclamide therapy in pre-clinical and/or clinical studies.
Conclusion
TBI and related cerebral edema are both heterogeneous processes with multiple, complex underlying cellular and molecular networks. We demonstrate that glibenclamide decreases diffuse cerebral edema at 24 h in the hemisphere contralateral to contusion in a combined injury model of CCI+HS. Edema in the contralateral hemisphere did not develop in CCI alone, and resolved by 72 h in combined injury. In contrast, glibenclamide failed to decrease edema in the ipsilateral hemisphere at 24 h or 72 h in either model. These results may reflect differences in injury levels, glibenclamide dose, treatment duration, and species compared with prior reports. Our findings suggest that the mechanisms underlying brain edema development may differ in injured versus contralateral hemispheres, with pericontusional edema seen in CCI alone (relatively refractory to our dose of glibenclamide), versus diffuse edema seen when CCI is complicated by HS and fluid resuscitation (highly responsive to our dose of glibenclamide). The TBI phenotype may, therefore, mandate precision-medicine approaches to treat brain edema. Although glibenclamide may not be able to replace therapies directed at decreasing contusional edema, it may be a useful and potentially preventative adjuvant to treat diffuse edema, particularly in patients with TBI and secondary insults requiring fluid resuscitation, and could be given in a preventative manner. Future studies exploring the effects of attenuating diffuse edema in the acute setting on long-term functional outcome in TBI are warranted.
Supplementary Material
Acknowledgments
We are grateful for National Institutes of Health (NIH) grants: T-32HL007820 (R.M.J.), KL2 TR000146 (R.M.J.), KL2-TR001856 (R.M.J.), K23NS101036 (R.M.J.), T32 HD040686 (J.S.W.), and R01 NS087978 (P.M.K.) for providing generous support. We are also grateful for United States Department of Defense grants W81XWH-10-1-0623 (P.M.K.) and W81XWH-14-2-0018 (P.M.K.) for providing generous support. We are also grateful to the UPP Foundation Grant for its generous support (RMJ).
Author Disclosure Statement
B.J.M. received research grants from Remedy Pharmaceuticals for clinical trial activities and was a site principal investigator for “Glyburide Advantage in Malignant Edema and Stroke (GAMES-RP)” a Remedy Pharmaceuticals funded study of glibenclamide for malignant edema in stroke. The other authors have nothing to disclose.
References
- 1.Faul M., and Coronado V. (2015). Epidemiology of traumatic brain injury. Handb. Clin. Neurol. 127, 3–13 [DOI] [PubMed] [Google Scholar]
- 2.Langlois J.A., Rutland-Brown W., and Wald M.M. (2006). The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 21, 375–378 [DOI] [PubMed] [Google Scholar]
- 3.Ghajar J. (2000). Traumatic brain injury. Lancet 356, 923–929 [DOI] [PubMed] [Google Scholar]
- 4.Chestnut R.M. (1995). Secondary brain insults after head injury: clinical perspectives. New Horiz. 3, 366–375 [PubMed] [Google Scholar]
- 5.Tisherman S.A., Schmicker R.H., Brasel K.J., Bulger E.M., Kerby J.D., Minei J.P., Powell J.L., Reiff D.A., Rizoli S.B., and Schreiber M.A. (2015). Detailed description of all deaths in both the shock and traumatic brain injury hypertonic saline trials of the Resuscitation Outcomes Consortium. Ann. Surg. 261, 586–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenberg H.M., Gary H.E., Aldrich E.F., Saydjari C., Turner B., Foulkes M.A., Jane J.A., Marmarou A., Marshall L.F., and Young H.F. (1990). Initial CT findings in 753 patients with severe head injury. A report from the NIH Traumatic Coma Data Bank. J. Neurosurg. 73, 688–698 [DOI] [PubMed] [Google Scholar]
- 7.Marmarou A. (2007). A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurg. Focus 22, E1. [DOI] [PubMed] [Google Scholar]
- 8.Ito J., Marmarou A., Barzó P., Fatouros P., and Corwin F. (1996). Characterization of edema by diffusion-weighted imaging in experimental traumatic brain injury. J. Neurosurg. 84, 97–103 [DOI] [PubMed] [Google Scholar]
- 9.Unterberg A.W., Stover J., Kress B., and Kiening K.L. (2004). Edema and brain trauma. Neuroscience 129, 1019–1027 [DOI] [PubMed] [Google Scholar]
- 10.Marmarou A., Signoretti S., Fatouros P.P., Portella G., Aygok G.A., and Bullock M.R. (2006). Predominance of cellular edema in traumatic brain swelling in patients with severe head injuries. J. Neurosurg. 104, 720–730 [DOI] [PubMed] [Google Scholar]
- 11.Chen J.-Q., Zhang C.-C., Jiang S.-N., Lu H., and Wang W. (2016). Effects of aquaporin 4 knockdown on brain edema of the uninjured side after traumatic brain injury in rats. Med. Sci. Monit. 22, 4809–4819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldmann H., Klages G., Gärtner F., and Scharfenberg J. (1979). The prognostic value of intracranial pressure monitoring after severe head injuries. Acta Neurochir. Suppl. (Wien) 28, 74–77 [DOI] [PubMed] [Google Scholar]
- 13.Feickert H.J., Drommer S., and Heyer R. (1999). Severe head injury in children: impact of risk factors on outcome. J. Trauma 47, 33–38 [DOI] [PubMed] [Google Scholar]
- 14.Miller J.D., Becker D.P., Ward J.D., Sullivan H.G., Adams W.E., and Rosner M.J. (1977). Significance of intracranial hypertension in severe head injury. J. Neurosurg. 47, 503–516 [DOI] [PubMed] [Google Scholar]
- 15.Saul T.G., and Ducker T.B. (1982). Effect of intracranial pressure monitoring and aggressive treatment on mortality in severe head injury. J. Neurosurg. 56, 498–503 [DOI] [PubMed] [Google Scholar]
- 16.Feinstein A.J., Patel M.B., Sanui M., Cohn S.M., Majetschak M., and Proctor K.G. (2005). Resuscitation with pressors after traumatic brain injury. J. Am. Coll. Surg. 201, 536–545 [DOI] [PubMed] [Google Scholar]
- 17.Bulger E.M., May S., Brasel K.J., Schreiber M., Kerby J.D., Tisherman S.A., Newgard C., Slutsky A., Coimbra R., Emerson S., Minei J.P., Bardarson B., Kudenchuk P., Baker A., Christenson J., Idris A., Davis D., Fabian T.C., Aufderheide T.P., Callaway C., Williams C., Banek J., Vaillancourt C., van Heest R., Sopko G., Hata J.S., Hoyt D.B., and ROC Investigators. (2010). Out-of-hospital hypertonic resuscitation following severe traumatic brain injury: a randomized controlled trial. JAMA 304, 1455–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.SAFE Study Investigators, Australian and New Zealand Intensive Care Society Clinical Trials Group, Australian Red Cross Blood Service, George Institute for International Health, Myburgh J., Cooper D.J., Finfer S., Bellomo R., Norton R., Bishop N., Kai Lo S., and Vallance S. (2007). Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N. Engl. J. Med. 357, 874–884 [DOI] [PubMed] [Google Scholar]
- 19.Simard J.M., Chen M., Tarasov K.V., Bhatta S., Ivanova S., Melnitchenko L., Tsymbalyuk N., West G.A., and Gerzanich V. (2006). Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat. Med. 12, 433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel A.D., Meriggioli M.N., Sanders D.B., Gerzanich V., Geng Z., and Simard J.M. (2010). Glibenclamide reduces hippocampal injury and preserves rapid spatial learning in a model of traumatic brain injury. J. Neuropathol. Exp. Neurol. 69, 1177–1190 [DOI] [PubMed] [Google Scholar]
- 21.Simard J.M., Kilbourne M., Tsymbalyuk O., Tosun C., Caridi J., Ivanova S., Keledjian K., Bochicchio G., and Gerzanich V. (2009). Key role of sulfonylurea receptor 1 in progressive secondary hemorrhage after brain contusion. J. Neurotrauma 26, 2257–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen M., Dong Y., and Simard J.M. (2003). Functional coupling between sulfonylurea receptor type 1 and a nonselective cation channel in reactive astrocytes from adult rat brain. J. Neurosci. 23, 8568–8577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woo S.K., Kwon M.S., Ivanov A., Gerzanich V., and Simard J.M. (2013). The sulfonylurea receptor 1 (Sur1)-transient receptor potential melastatin 4 (Trpm4) channel. J. Biol. Chem. 288, 3655–3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen M., and Simard J.M. (2001). Cell swelling and a nonselective cation channel regulated by internal Ca2+ and ATP in native reactive astrocytes from adult rat brain. J. Neurosci. 21, 6512–6521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Portella G., Beaumont A., Corwin F., Fatouros P., and Marmarou A. (2000). Characterizing edema associated with cortical contusion and secondary insult using magnetic resonance spectroscopy. Acta Neurochir. Suppl. 76, 273–275 [DOI] [PubMed] [Google Scholar]
- 26.Dennis A.M., Haselkorn M.L., Vagni V.A., Garman R.H., Janesko-Feldman K., Bayır H., Clark R.S.B., Jenkins L.W., Dixon C.E., and Kochanek P.M. (2009). Hemorrhagic shock after experimental traumatic brain injury in mice: effect on neuronal death. J. Neurotrauma 26, 889–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brockman E.C., Bayir H., Blasiole B., Shein S.L., Fink E.L., Dixon C., Clark R.S., Vagni V.A., Ma L., Hsia C.J., Tisherman S.A., and Kochanek P.M. (2013). Polynitroxylated-pegylated hemoglobin attenuates fluidrequirements and brain edema in combined traumatic brain injuryplus hemorrhagic shock in mice. J. Cereb. Blood Flow Metab. 33, 1457–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shellington D.K., Du L., Wu X., Exo J., Vagni V., Ma L., Janesko-Feldman K., Clark R.S.B., Bayir H., Dixon C.E., Jenkins L.W., Hsia C.J.C., and Kochanek P.M. (2011). Polynitroxylated pegylated hemoglobin: a novel neuroprotective hemoglobin for acute volume-limited fluid resuscitation after combined traumatic brain injury and hemorrhagic hypotension in mice. Crit. Care Med. 39, 494–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hemerka J.N., Wu X., Dixon C.E., Garman R.H., Exo J.L., Shellington D.K., Blasiole B., Vagni V.A., Janesko-Feldman K., Xu M., Wisniewski S.R., Bayır H., Jenkins L.W., Clark R.S.B., Tisherman S.A., and Kochanek P.M. (2012). Severe brief pressure-controlled hemorrhagic shock after traumatic brain injury exacerbates functional deficits and long-term neuropathological damage in mice. J. Neurotrauma 29, 2192–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simard J.M., Kilbourne M., Tsymbalyuk O., Tosun C., Caridi J., Ivanova S., Keledjian K., Bochicchio G., and Gerzanich V. (2009). Key role of sulfonylurea receptor 1 in progressive secondary hemorrhage after brain contusion. J. Neurotrauma 26, 2257–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zweckberger K., Hackenberg K., Jung C.S., Hertle D.N., Kiening K.L., Unterberg A.W., and Sakowitz O.W. (2014). Glibenclamide reduces secondary brain damage after experimental traumatic brain injury. Neuroscience 272, 199–206 [DOI] [PubMed] [Google Scholar]
- 32.Naraharisetti S.B., Kirby B.J., Hebert M.F., Easterling T.R., and Unadkat J.D. (2007). Validation of a sensitive LC-MS assay for quantification of glyburide and its metabolite 4-transhydroxy glyburide in plasma and urine: an OPRU Network study. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 860, 34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mansfield R.T., Schiding J.K., Hamilton R.L., and Kochanek P.M. (1996). Effects of hypothermia on traumatic brain injury in immature rats. J. Cereb. Blood Flow Metab. 16, 244–252 [DOI] [PubMed] [Google Scholar]
- 34.Simard J.M., Yurovsky V., Tsymbalyuk N., Melnichenko L., Ivanova S., and Gerzanich V. (2009). Protective effect of delayed treatment with low-dose glibenclamide in three models of ischemic stroke. Stroke 40, 604–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simard J.M., Popovich P.G., Tsymbalyuk O., and Gerzanich V. (2012). Spinal cord injury with unilateral versus bilateral primary hemorrhage- effects of glibenclamide. Exp. Neurol. 233, 829–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chestnut R.M., Marshall L.F., Klauber M.R., Blunt B.A., Baldwin N., Eisenberg H.M., Jane J.A., Marmarou A., and Foulkes M.A. (1993). The role of secondary brain injury in determining outcome from severe head injury. J. Trauma 34, 216–222 [DOI] [PubMed] [Google Scholar]
- 37.Stiefel M.F., Tomita Y., and Marmarou A. (2005). Secondary ischemia impairing the restoration of ion homeostasis following traumatic brain injury. J. Neurosurg. 103, 707–714 [DOI] [PubMed] [Google Scholar]
- 38.Katayama Y., Mori T., Maeda T., and Kawamata T. (1998). Pathogenesis of the mass effect of cerebral contusions: rapid increase in osmolality within the contusion necrosis. Acta Neurochir. Suppl. 71, 289–292 [DOI] [PubMed] [Google Scholar]
- 39.Katayama Y., and Kawamata T. (2003). Edema fluid accumulation within necrotic brain tissue as a cause of the mass effect of cerebral contusion in head trauma patients. Acta Neurochir. Suppl. (Wien) 86, 323–327 [DOI] [PubMed] [Google Scholar]
- 40.Xu Z.-M., Yuan F., Liu Y.-L., Ding J., and Tian H.-L. (2016). Glibenclamide attenuates blood-brain barrier disruption in adult mice after traumatic brain injury. J. Neurotrauma 15, 925–933 [DOI] [PubMed] [Google Scholar]
- 41.Shigemori Y., Katayama Y., Mori T., Maeda T., and Kawamata T. (2006). Matrix metalloproteinase-9 is associated with blood-brain barrier opening and brain edema formation after cortical contusion in rats. Acta Neurochir. Suppl. 96, 130–133 [DOI] [PubMed] [Google Scholar]
- 42.Hadass O., Tomlinson B.N., Gooyit M., Chen S., Purdy J.J., Walker J.M., Zhang C., Giritharan A.B., Purnell W., Robinson C.R., Shin D., Schroeder V.A., Suckow M.A., Simonyi A., Sun G.Y., Mobashery S., Cui J., Chang M., and Gu Z. (2013). Selective inhibition of matrix metalloproteinase-9 attenuates secondary damage resulting from severe traumatic brain injury. PLoS One 8, e76904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimbler D.E., Shields J., Yanasak N., Vender J.R., and Dhandapani K.M. (2012). Activation of P2X7 promotes cerebral edema and neurological injury after traumatic brain injury in mice. PLoS One 7, e41229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiening K.L., van Landeghem F.K.H., Schreiber S., Thomale U.W., Deimling , von A., Unterberg A.W., and Stover J.F. (2002). Decreased hemispheric Aquaporin-4 is linked to evolving brain edema following controlled cortical impact injury in rats. Neurosci. Lett. 324, 105–108 [DOI] [PubMed] [Google Scholar]
- 45.Lopez-Rodriguez A.B., Acaz-Fonseca E., Viveros M.-P., and Garcia-Segura L.M. (2015). Changes in cannabinoid receptors, aquaporin 4 and vimentin expression after traumatic brain injury in adolescent male mice. Association with edema and neurological deficit. PLoS One 10, e0128782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang F., Luo C., Xu G., Su F., He X., Long S., Ren H., Liu Y., Feng Y., and Pei Z. (2015). Deletion of aquaporin-4 is neuroprotective during the acute stage of micro traumatic brain injury in mice. Neurosci. Lett. 598, 29–35 [DOI] [PubMed] [Google Scholar]
- 47.Yao X., Uchida K., Papadopoulos M.C., Zador Z., Manley G.T., and Verkman A.S. (2015). Mildly reduced brain swelling and improved neurological outcome in aquaporin-4 knockout mice following controlled cortical impact brain injury. J. Neurotrauma 32, 1458–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laird M.D., Shields J.S., Sukumari-Ramesh S., Kimbler D.E., Fessler R.D., Shakir B., Youssef P., Yanasak N., Vender J.R., and Dhandapani K.M. (2014). High mobility group box protein-1 promotes cerebral edema after traumatic brain injury via activation of toll-like receptor 4. Glia 62, 26–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okuma Y., Liu K., Wake H., Zhang J., Maruo T., Date I., Yoshino T., Ohtsuka A., Otani N., Tomura S., Shima K., Yamamoto Y., Yamamoto H., Takahashi H.K., Mori S., and Nishibori M. (2012). Anti-high mobility group box-1 antibody therapy for traumatic brain injury. Ann. Neurol. 72, 373–384 [DOI] [PubMed] [Google Scholar]
- 50.Walcott B.P., Kahle K.T., and Simard J.M. (2011). Novel treatment targets for cerebral edema. Neurotherapeutics 9, 65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jayakumar A.R., Panickar K.S., Curtis K.M., Tong X.Y., Moriyama M., and Norenberg M.D. (2011). Na-K-Cl cotransporter-1 in the mechanism of cell swelling in cultured astrocytes after fluid percussion injury. J. Neurochem. 117, 437–448 [DOI] [PubMed] [Google Scholar]
- 52.Simard J.M., Kahle K.T., and Gerzanich V. (2010). Molecular mechanisms of microvascular failure in central nervous system injury—synergistic roles of NKCC1 and SUR1/TRPM4. J. Neurosurg. 113, 622–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Langfitt T.W., Weinstein J.D., and Kassell N.F. (1964). Cerebral vasomotor paralysis as a cause of brain swelling. Trans. Am. Neurol. Assoc. 89, 214–215 [PubMed] [Google Scholar]
- 54.Langfitt T.W. (1966). Etiology and management of acute brain swelling. W. V. Med. J. 62, 49. [PubMed] [Google Scholar]
- 55.Langfitt T.W., Tannanbaum H.M., and Kassell N.F. (1966). The etiology of acute brain swelling following experimental head injury. J. Neurosurg. 24, 47–56 [DOI] [PubMed] [Google Scholar]
- 56.Schutta H.S., Kassell N.F., and Langfitt T.W. (1968). Brain swelling produced by injury and aggravated by arterial hypertension. A light and electron microscopic study. Brain 91, 281–294 [DOI] [PubMed] [Google Scholar]
- 57.Marmarou A., Fatouros P.P., Barzó P., Portella G., Yoshihara M., Tsuji O., Yamamoto T., Laine F., Signoretti S., Ward J.D., Bullock M.R., and Young H.F. (2000). Contribution of edema and cerebral blood volume to traumatic brain swelling in head-injured patients. J. Neurosurg. 93, 183–193 [DOI] [PubMed] [Google Scholar]
- 58.Klatzo I. (1994). Evolution of brain edema concepts, in: Brain Edema IX. Springer Vienna: Vienna, pps. 3–6 [DOI] [PubMed] [Google Scholar]
- 59.Simard J.M., Woo S.K., Schwartzbauer G.T., and Gerzanich V. (2012). Sulfonylurea receptor 1 in central nervous system injury: a focused review. J. Cereb. Blood Flow Metab. 32, 1699–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whalen M.J., Carlos T.M., Kochanek P.M., Clark R.S., Heineman S., Schiding J.K., Franicola D., Memarzadeh F., Lo W., Marion D.W., and DeKosky S.T. (1999). Neutrophils do not mediate blood-brain barrier permeability early after controlled cortical impact in rats. J. Neurotrauma 16, 583–594 [DOI] [PubMed] [Google Scholar]
- 61.Simard J.M., Kent T.A., Chen M., Tarasov K.V., and Gerzanich V. (2007). Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 6, 258–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krieg S.M., Voigt F., Knuefermann P., Kirschning C.J., Plesnila N., and Ringel F. (2017). Decreased secondary lesion growth and attenuated immune response after traumatic brain injury in Tlr2/4(-/-) mice. Front. Neurol. 8, 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teng Z., Guo Z., Zhong J., Cheng C., Huang Z., Wu Y., Tang S., Luo C., Peng X., Wu H., Sun X., and Jiang L. (2017). ApoE influences the blood-brain barrier through the NF-κB/MMP-9 pathway after traumatic brain injury. Sci. Rep. 7, 6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krieg S.M., Trabold R., and Plesnila N. (2017). Time-dependent effects of arginine-vasopressin v1 receptor inhibition on secondary brain damage after traumatic brain injury. J. Neurotrauma 34, 1329–1336 [DOI] [PubMed] [Google Scholar]
- 65.Kochanek P.M., Marion D.W., Zhang W., Schiding J.K., White M., Palmer A.M., Clark R.S., O'Malley M.E., Styren S.D., and Ho C. (1995). Severe controlled cortical impact in rats: assessment of cerebral edema, blood flow, and contusion volume. J. Neurotrauma 12, 1015–1025 [DOI] [PubMed] [Google Scholar]
- 66.Zhang M., Cui Z., Cui H., Cao Y., Zhong C., and Wang Y. (2016). Astaxanthin alleviates cerebral edema by modulating NKCC1 and AQP4 expression after traumatic brain injury in mice. BMC Neurosci. 17, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nagata K., Kumasaka K., Browne K.D., Li S., St-Pierre J., Cognetti J., Marks J., Johnson V.E., Smith D.H., and Pascual J.L. (2016). Unfractionated heparin after TBI reduces in vivo cerebrovascular inflammation, brain edema and accelerates cognitive recovery. J. Trauma Acute Care Surg. 81, 1088–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marmarou C.R., Liang X., Abidi N.H., Parveen S., Taya K., Henderson S.C., Young H.F., Filippidis A.S., and Baumgarten C.M. (2014). Selective vasopressin-1a receptor antagonist prevents brain edema, reduces astrocytic cell swelling and GFAP, V1aR and AQP4 expression after focal traumatic brain injury. Brain Res. 1581, 89–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krieg S.M., Sonanini S., Plesnila N., and Trabold R. (2015). Effect of small molecule vasopressin V1a and V2 receptor antagonists on brain edema formation and secondary brain damage following traumatic brain injury in mice. J. Neurotrauma 32, 221–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McBride D.W., Szu J.I., Hale C., Hsu M.S., Rodgers V.G.J., and Binder D.K. (2014). Reduction of cerebral edema after traumatic brain injury using an osmotic transport device. J. Neurotrauma 31, 1948–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kenne E., Erlandsson A., Lindbom L., Hillered L., and Clausen F. (2012). Neutrophil depletion reduces edema formation and tissue loss following traumatic brain injury in mice. J. Neuroinflammation 9, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tress E.E., Clark R.S.B., Foley L.M., Alexander H., Hickey R.W., Drabek T., Kochanek P.M., and Manole M.D. (2014). Blood brain barrier is impermeable to solutes and permeable to water after experimental pediatric cardiac arrest. Neurosci. Lett. 578, 17–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jha R.M., Yan H., Dixon C.E., Poloyac S., Jackson T., Hoshitsuki K., Ma X., Henchir J., and Kochanek P.M. (2015). Evaluation of glibenclamide in the Pittsburgh Controlled Cortical Impact Model of Traumatic Brain Injury: an OBTT consortium study. J. Neurotrauma 32, 119–152 [Google Scholar]
- 74.Jha R., Puccio A., Chou S., Chang C.-C., Wallisch J., Molyneaux B., Zusman B., Shutter L., Poloyac S., Keri J.-F., Okonkwo D., and Kochanek P. (2016). Sulfonylurea receptor-1 as a novel biomarker for cerebral edema in patients with severe traumatic brain injury (S46.001). Crit. Care Med. 45, e255–e264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simard J.M., Woo S.K., Norenberg M.D., Tosun C., Chen Z., Ivanova S., Tsymbalyuk O., Bryan J., Landsman D., and Gerzanich V. (2010). Brief suppression of Abcc8 Prevents autodestruction of spinal cord after trauma. Sci. Transl. Med. 2, 28ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Munoz A., Nakazaki M., Goodman J.C., Barrios R., Onetti C.G., Bryan J., and Aguilar-Bryan L. (2003). Ischemic preconditioning in the hippocampus of a knockout mouse lacking SUR1-based KATP channels. Stroke 34, 164–170 [DOI] [PubMed] [Google Scholar]
- 77.Simard J.M., Geng Z., Woo S.K., Ivanova S., Tosun C., Melnichenko L., and Gerzanich V. (2009). Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 29, 317–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simard J.M., Tsymbalyuk N., Tsymbalyuk O., Ivanova S., Yurovsky V., and Gerzanich V. (2010). Glibenclamide is superior to decompressive craniectomy in a rat model of malignant stroke. Stroke 41, 531–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang K., Gu Y., Hu Y., Ji Z., Wang S., Lin Z., Li X., Xie Z., and Pan S. (2015). Glibenclamide improves survival and neurological outcome after cardiac arrest in rats. Crit. Care Med. 43, e341–e349 [DOI] [PubMed] [Google Scholar]
- 80.Ortega F.J., Jolkkonen J., Mahy N., and guez M.J.R.I. (2012). Glibenclamide enhances neurogenesis and improveslong-term functional recovery after transient focal cerebralischemia. J. Cereb. Blood Flow Metab. 33, 356–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ortega F.J., Gimeno-Bayon J., Espinosa-Parrilla J.F., Carrasco J.L., Batlle M., Pugliese M., Mahy N., and Rodríguez M.J. (2012). Contemporary management of spinal AVFs and AVMs: lessons learned from 110 cases. Exp. Neurol. 235, 282–296 [DOI] [PubMed] [Google Scholar]
- 82.Simard J.M., Sheth K.N., Kimberly W.T., Stern B.J., del Zoppo G.J., Jacobson S., and Gerzanich V. (2014). Glibenclamide in cerebral ischemia and stroke. Neurocrit. Care 20, 319–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schattling B., Steinbach K., Thies E., Kruse M., Menigoz A., Ufer F., Flockerzi V., Brück W., Pongs O., Vennekens R., Kneussel M., Freichel M., Merkler D., and Friese M.A. (2012). TRPM4 cation channel mediates axonal and neuronal degeneration in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat. Med. 18, 1805–1811 [DOI] [PubMed] [Google Scholar]
- 84.Makar T.K., Gerzanich V., Nimmagadda V.K.C., Jain R., Lam K., Mubariz F., Trisler D., Ivanova S., Woo S.K., Kwon M.S., Bryan J., Bever C.T., and Simard J.M. (2015). Silencing of Abcc8 or inhibition of newly upregulated Sur1-Trpm4 reduce inflammation and disease progression in experimental autoimmune encephalomyelitis. J Neuroinflammation 12, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zafardoost P., Ghasemi A.A., Salehpour F., Piroti C., and Ziaeii E. (2016). Evaluation of the effect of glibenclamide in patients with diffuse axonal injury due to moderate to severe head trauma. Trauma Mon. 21, e25113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.