Abstract
Evening dosing of antihypertensive medications lowers nighttime blood pressure and, in one large randomized trial, reduced risk for cardiovascular outcomes. However, feasibility of nighttime dosing in routine clinical practice is unknown. The purpose of this pilot study was to evaluate the effect of a brief pharmacist intervention to assign patients to take antihypertensive medications at specific times of the day. In this pilot, randomized controlled trial, 79 patients with moderate to severe CKD taking one or more antihypertensive medications once daily were randomized to take one once daily antihypertensive either in the morning or the evening. A total of 79 patients were randomized (39 to morning dosing, 40 to evening dosing). Average (SD) age was 56.5 (14) years, 68% were male, and average (SD) estimated glomerular filtration rate was 36.6 (8.9) mL/min/1.73m2. Adherence, defined as taking the once daily medication at the time indicated 6 or 7 times in the last 7 days and not taking it at any other time during the day, was 91% in the morning arm and 95% in the evening arm (P=0.57). This pilot demonstrates the feasibility and efficacy of a pharmacist-physician collaborative to assign one-daily antihypertensive medications to either morning or evening dosing.
Keywords: Hypertension, Adherence, Chronic Kidney Disease, Chronotherapy
Background
Hypertension is a major risk factor for cardiovascular and renal disease and is often associated with premature mortality worldwide. Previous trials have demonstrated that treatment of hypertension reduces the risk for cardiovascular disease and all-cause mortality (1,2). Nearly all prior studies have investigated the effect of reduction in clinic blood pressure rather than in ambulatory blood pressure. Clinic blood pressures are taken during daytime hours, while ambulatory blood pressure monitoring allows for the assessment of blood pressure throughout the day and night (3). Nighttime systolic blood pressure, which can be lowered through evening dosing of antihypertensive medications, has been shown to be predictive of cardiovascular disease and all-cause mortality (2–4), as well as the risk of renal events (5,6). Additionally, nighttime blood pressure is elevated in patients with CKD and proteinuria (7). In one large, single center, randomized controlled trial, evening dosing of antihypertensive medications significantly reduced the risk of cardiovascular events (8), including among those with CKD (9).
Pragmatic randomized controlled trials are currently being planned or are underway to evaluate whether evening dosing of antihypertensives reduces adverse outcomes (10,11). However, the feasibility of evening dosing in routine clinical practice is unknown. Studies have demonstrated pharmacist-physician collaborative interventions have successfully reduced nighttime blood pressure (12,13). Research has also examined the value of pharmacist-based interventions to improve medication adherence, and has illustrated that pharmacists play a unique role in improving blood pressure control in patients with and without CKD (14–21). These studies suggest that a pharmacist-physician collaborative may be a feasible strategy to implement evening dosing of antihypertensive medications, although there is little data regarding how these interventions affect patient adherence to their once daily medication.
Objective:
We conducted a pilot study to evaluate the feasibility of a brief pharmacist intervention to assign antihypertensive medications to morning versus evening dosing and examine the effect on short-term medication adherence.
Methods
Study Design and Patient Population
This study was a prospective, randomized trial of patients visiting the University of Minnesota Physicians (UMP) Nephrology Clinic from January 2014 to April 2015. Patients aged 18–90 years of age who were taking one or more once-daily non-diuretic antihypertensive medications were identified from their electronic health record. Only patients with moderate to severe kidney disease were included. Specifically, we included those with an estimated glomerular filtration rate (eGFR) of 20–45 ml/min/1.73m2 or those with an eGFR of 45–60 ml/min/1.73m2 who also had proteinuria, defined by either a urine albumin to creatinine ratio >300mg/g or a urine protein to creatinine ratio >500mg/g. Pregnant women and those with conditions or characteristics that could inhibit follow-up and/or medication adherence (e.g., non-English speaking, disabled) were excluded.
A student pharmacist approached eligible patients in their exam room at their next nephrology clinic visit and asked if they were interested in participating in the study. If the student pharmacist was unable to contact the patient at the time of the clinic visit, the student called the patient and consent was obtained over the phone. Informed consent was obtained from all participants. The University of Minnesota’s Institutional Review Board approved the protocol for this study.
Intervention – Study Groups
Participants were randomized 1:1 in computer generated blocks of 2, 4, and 6 to one of two antihypertensive dosing treatment groups (morning or evening). The student pharmacist reviewed each participant’s antihypertensive medications with a focus on the once-daily antihypertensive assigned to the morning or evening study group. The student pharmacist gave each participant a personal medication record with specific instructions regarding the once-daily antihypertensive medication assigned to morning or evening dosing. If a patient was taking more than one antihypertensive medication, only one was used for the current study, with priority given to angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), then to calcium channel blockers (CCB), beta-blockers, and then finally to other non-diuretic antihypertensive medications. Patients taking only a diuretic were not eligible for this trial since evening dosing may increase overnight urination and pose an unnecessary burden on the subject.
Study Endpoints
Participants were contacted by phone three to six weeks after their clinic visit by the student pharmacist to assess general medication adherence utilizing the Morisky Medication Scale (22). Participants were also asked two questions regarding the antihypertensive medication assigned to morning or evening dosing: 1) how many times in the last seven days they took the medication in the morning, and 2) how many times in the last seven days they took the medication in the evening (see supplemental material for the full medication adherence survey script).
The primary clinical outcome was adherence to the instructions regarding timing of dosing of the study specific antihypertensive medication. Adherence was defined as taking the once daily medication at the time indicated 6 or 7 times in the last 7 days and not taking it at any other time during the day.
Statistical Analysis
Baseline characteristics are described using means and standard deviations, as well as counts and percentages. The difference in the rate of adherence between the morning and evening groups was assessed using a chi-square test.
Results
A total of 99 eligible patients were identified at the UMP Nephrology Clinic between January 2014 and April 2015. All participants met the inclusion and exclusion criteria and were approached in clinic. Eighteen patients declined to participate in the study prior to randomization, and 2 patients had medication changes at the index visit and were unable to participate. A total of 79 patients were randomized: 39 into the morning dosing arm and 40 into the evening dosing arm. Baseline patient characteristics are shown in Table 1. Average (SD) age was 56.5 (14) years, 68% were male, and the average (SD) eGFR was 36.6 (8.9) ml/min/1.73m2. Study medications included: 49 ACEI/ARB, 28 calcium channel blockers, and 2 beta-blockers. Despite three attempts, 5 patients in the morning group and 3 patients in the evening group were lost to follow-up. Therefore, the final analysis cohort included 34 participants in the morning group and 37 participants in the evening group (Figure 1).
Table 1:
Baseline Characteristics
| Morning arm (N=39 patients) |
Evening arm (N=40 patients) |
|
|---|---|---|
| Demographics | ||
| Age, mean (SD), years | 56.1 (13.3) | 56.9 (14.7) |
| Male Sex, N (%) | 28 (72%) | 26 (65%) |
| Clinical Characteristics | ||
| eGFR, mean (SD), mL/min/1.73m2 | 36.4 (7.4) | 36.9 (10.2) |
| eGFR category, N (%) | ||
| ≥ 45 mL/min/1.73m2 | 3 (7.7%) | 6 (15%) |
| 30 to 45 mL/min/1.73m2 | 29 (74.4%) | 20 (50%) |
| < 30 mL/min/1.73m2 | 7 (17.9%) | 14 (35%) |
| Proteinuria, N (%) | 25 (66%) | 22 (56%) |
| Diabetes, N (%) | 20 (51.3%) | 13 (32.5%) |
| Coronary artery disease, N (%) | 7 (17.9%) | 4 (10%) |
| Study medication | ||
| ACEI/ARB, N (%) | 22 (56.4%) | 27 (67.5%) |
| Calcium channel blocker, N (%) | 16 (41.0%) | 12 (30.0%) |
| Beta-blocker, N (%) | 1 (2.6%) | 1 (2.5%) |
ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; eGFR: estimated glomerular filtration rate; SD: standard deviation
Figure 1:
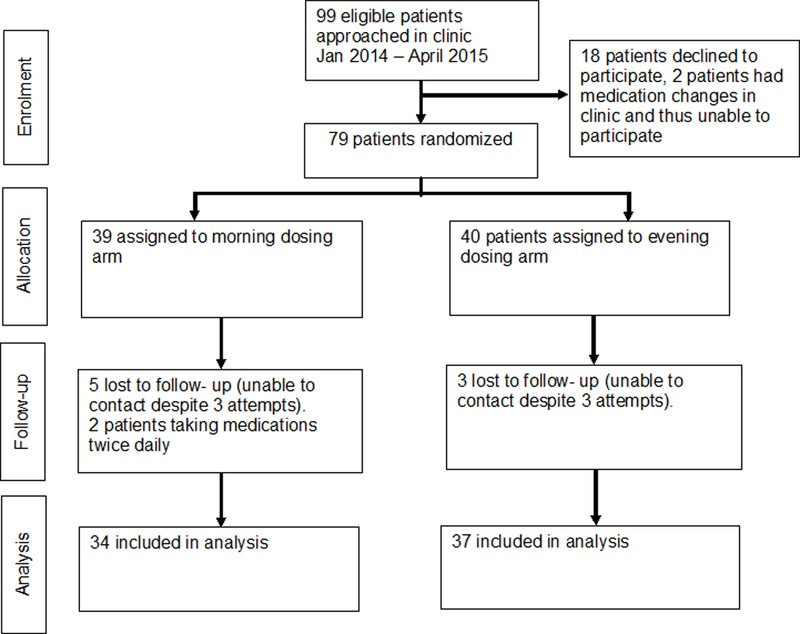
CONSORT: Trial Process Flow
The mean (SD) time to follow-up was 29 (6) days; there was no difference between study arms. Study participants reported excellent adherence as assessed by the Morisky Medication Scale, with no difference between treatment arms. Adherence to the timing of the once daily antihypertensive medication was 91% in the morning arm and 95% in the evening arm (P=0.57, Table 2). Among those who were non-adherent, 2 patients in the morning dosing group were taking medications at night, and 1 patient in the evening group was taking medications in the morning. The average (SD) time spent by the pharmacy student with the participants was 9.9 (6.1) minutes (interquartile range 6 to 12 minutes), and the average (SD) time from the clinic to follow up call was 29.1 (6.1) days.
Table 2:
Adherence by Treatment Arm, Patients Contacted for Follow-Up Survey
| Variable | Morning arm (N=34) |
Evening arm (N=37) |
|---|---|---|
| Adherent,a n (%) | 31 (91.2%) | 35 (94.6%)b |
| Morning doses in the last 7 days | ||
| 0 doses | 0 | 36 |
| 5 doses | 1c | 0 |
| 6 doses | 4 | 0 |
| 7 doses | 29 | 1c |
| Evening doses in the last 7 days | ||
| 0 doses | 32 | 1c |
| 4 doses | 1c | 0 |
| 5 doses | 0 | 1c |
| 6 doses | 1c | 0 |
| 7 doses | 0 | 35 |
Taking medication at time indicated 6 or 7 times in the last 7 days and not taking at other time during the day.
Chi-squared p-value for comparison of percent adherent in morning vs. evening arm = 0.57.
Represents an event that would indicate non-adherence.
Discussion
This randomized controlled trial demonstrates that a brief pharmacist based intervention is effective in the short term for assigning once-daily antihypertensive medications to morning or evening dosing in a CKD patient population. The intervention proved feasible in the routine clinic setting: the average time spent by the pharmacist with each participant was less than 10 minutes. Further, this time included obtaining patient consent, so the time required to implement this program in the routine clinic setting may be even less. These results suggest that implementing evening dosing in the clinic setting is possible.
Nighttime systolic blood pressure may be the best predictor of cardiovascular disease and mortality (2); one prior study has shown that evening dosing of a single once daily antihypertensive medication may reduce adverse cardiovascular outcomes (2,3,8,9). Hermida et al. studied 2156 randomized subjects to demonstrate significant effects of administration time, specifically a lower CVD event risk (i.e., death, myocardial infarction, stroke) (8). Among a small subset of subjects (N=661) with CKD, evening dosing was associated with lower risks of cardiovascular death, myocardial infraction and stroke during a median follow-up of 5.4 years (9). There is evidence that nighttime systolic blood pressure is higher among CKD patients (23), possibly due to altered diurnal variation in angiotensinogen excretion that can affect the circadian rhythm of both the intrarenal renin-angiotensin system and blood pressure (24). Since nighttime blood pressure and non-dipping are potential modifiable risk factors in patients with CKD (2), the evening dosing of antihypertensive medications may have an impact on the progression of CKD. Further research is needed to evaluate the impact of lowering nighttime blood pressure on CKD progression.
When considering any change for how patients take their medication, providers should consider whether there could be any impact on adherence, as patients with poor medication adherence have been found to be more likely to experience chronic disease state progression (25). Although adherence to dosing did not differ between the morning and evening groups in the current study, a previous study assessed electronically compiled dosing histories to investigate adherence to prescribed antihypertensive medications in 4783 patients and found that patients were more likely to take their medications as prescribed in the morning than in the evening (26). Previous studies have demonstrated that pharmacist-based interventions can improve adherence in both the CKD patient population and in primary care (14–21,27). The results of the current study further support the notion that pharmacist-based interventions can improve adherence in chronic patient disease populations, but as identified in previous clinical studies, further research must be conducted to evaluate the impact of adherence on long-term clinical outcomes (21,27).
This study has limitations that should be considered. A full medication therapy management visit was not conducted. The student pharmacist, who conducted the trial under the supervision of a nephrologist and registered pharmacist, reviewed all patients’ antihypertensive medications, but the main focus was on the study specific antihypertensive medication. However, the study did demonstrate excellent short-term adherence to this brief intervention. Another limitation is that there was no adjustment for the total number of medications taken or the duration of time the patient had been taking the study specific medication. Further, our data reflected much higher levels of adherence than has been reported in literature for other chronic conditions (55–73%) (19,28). These results may be due to the method of measuring adherence (patient reported) and the phrasing of the first question on the Medication Adherence Script (Supplemental Material). Although the Morisky Medication Scale has been shown to be a reliable tool to assess patient adherence (22), self-reported data may overestimate true adherence. Finally, this study does not establish the sustainability of this intervention over time.
Conclusion
This pilot demonstrates the feasibility and efficacy of a pharmacist-physician collaborative to assign once-daily antihypertensive medications to either morning or evening dosing. A high percentage of patients were willing to participate in the study, and adherence to the assigned dosing time was high in both the morning and evening groups. The data supports the efficacy associated with pharmacist based antihypertensive medication review and follow-up in the CKD patient population. These results will help inform the design of large pragmatic clinical trials evaluating whether evening dosing of antihypertensive medications improves clinical outcomes.
Acknowledgements
The authors would like to acknowledge Craig Solid of Solid Research Group, LLC for assistance in preparation and formatting of this manuscript. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number 8UL1TR000114. The content was solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors have no conflicts of interests, financial or otherwise, to disclose.
References
- 1.James PA, Oparil S, Carter BL, et al. 2014. evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA February 05 2014;311(5):507–520. [DOI] [PubMed] [Google Scholar]
- 2.Mehta R, Drawz PE. Is nocturnal blood pressure reduction the secret to reducing the rate of progression of hypertensive chronic kidney disease? Curr Hypertens Rep October 2011;13(5):378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension January 2011;57(1):3–10. [DOI] [PubMed] [Google Scholar]
- 4.Minutolo R, Gabbai FB, Borrelli S, et al. Changing the timing of antihypertensive therapy to reduce nocturnal blood pressure in CKD: an 8-week uncontrolled trial. Am J Kidney Dis December 2007;50(6):908–917. [DOI] [PubMed] [Google Scholar]
- 5.Drawz PE, Rosenthal N, Babineau DC, Rahman M. Nighttime hospital blood pressure--a predictor of death, ESRD, and decline in GFR. Ren Fail 2010;32(9):1036–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redon J, Plancha E, Swift PA, Pons S, Munoz J, Martinez F. Nocturnal blood pressure and progression to end-stage renal disease or death in nondiabetic chronic kidney disease stages 3 and 4. J Hypertens March 2010;28(3):602–607. [DOI] [PubMed] [Google Scholar]
- 7.Drawz PE, Alper AB, Anderson AH, et al. Masked Hypertension and Elevated Nighttime Blood Pressure in CKD: Prevalence and Association with Target Organ Damage. Clin J Am Soc Nephrol April 07 2016;11(4):642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int September 2010;27(8):1629–1651. [DOI] [PubMed] [Google Scholar]
- 9.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Bedtime dosing of antihypertensive medications reduces cardiovascular risk in CKD. J Am Soc Nephrol December 2011;22(12):2313–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter BL, Chrischilles EA, Rosenthal G, Gryzlak BM, Eisenstein EL, Vander Weg MW. Efficacy and safety of nighttime dosing of antihypertensives: review of the literature and design of a pragmatic clinical trial. J Clin Hypertens (Greenwich) February 2014;16(2):115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rorie DA, Rogers A, Mackenzie IS, et al. Methods of a large prospective, randomised, open-label, blinded end-point study comparing morning versus evening dosing in hypertensive patients: the Treatment In Morning versus Evening (TIME) study. BMJ Open February 09 2016;6(2):e010313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter BL, Bergus GR, Dawson JD, et al. A cluster randomized trial to evaluate physician/pharmacist collaboration to improve blood pressure control. J Clin Hypertens (Greenwich) April 2008;10(4):260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber CA, Ernst ME, Sezate GS, Zheng S, Carter BL. Pharmacist-physician comanagement of hypertension and reduction in 24-hour ambulatory blood pressures. Arch Intern Med October 11 2010;170(18):1634–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albsoul-Younes AM, Hammad EA, Yasein NA, Tahaineh LM. Pharmacist-physician collaboration improves blood pressure control. Saudi Med J March 2011;32(3):288–292. [PubMed] [Google Scholar]
- 15.Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA July 03 2013;310(1):46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.St Peter WL, Farley TM, Carter BL. Role of collaborative care models including pharmacists in improving blood pressure management in chronic kidney disease patients. Curr Opin Nephrol Hypertens September 2011;20(5):498–503. [DOI] [PubMed] [Google Scholar]
- 17.St Peter WL, Wazny LD, Patel UD. New models of chronic kidney disease care including pharmacists: improving medication reconciliation and medication management. Curr Opin Nephrol Hypertens November 2013;22(6):656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch JD, Steers N, Adler DS, et al. Primary care-based, pharmacist-physician collaborative medication-therapy management of hypertension: a randomized, pragmatic trial. Clin Ther September 01 2014;36(9):1244–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mino-Leon D, Reyes-Morales H, Flores-Hernandez S. Effectiveness of involving pharmacists in the process of ambulatory health care to improve drug treatment adherence and disease control. J Eval Clin Pract February 2015;21(1):7–12. [DOI] [PubMed] [Google Scholar]
- 20.Zillich AJ, Jaynes HA, Bex SD, et al. Evaluation of pharmacist care for hypertension in the Veterans Affairs patient-centered medical home: a retrospective case-control study. Am J Med May 2015;128(5):539 e531-536. [DOI] [PubMed] [Google Scholar]
- 21.Cooney D, Moon H, Liu Y, et al. A pharmacist based intervention to improve the care of patients with CKD: a pragmatic, randomized, controlled trial. BMC Nephrol April 16 2015;16:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) May 2008;10(5):348–354. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Ruiz-Hurtado G, Ruilope LM, de la Sierra A, et al. Association Between High and Very High Albuminuria and Nighttime Blood Pressure: Influence of Diabetes and Chronic Kidney Disease. Diabetes Care October 2016;39(10):1729–1737. [DOI] [PubMed] [Google Scholar]
- 24.Ohashi N, Isobe S, Ishigaki S, Yasuda H. Circadian rhythm of blood pressure and the renin-angiotensin system in the kidney. Hypertens Res December 01 2016. [DOI] [PubMed] [Google Scholar]
- 25.Dragomir A, Cote R, Roy L, et al. Impact of adherence to antihypertensive agents on clinical outcomes and hospitalization costs. Med Care May 2010;48(5):418–425. [DOI] [PubMed] [Google Scholar]
- 26.Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ May 17 2008;336(7653):1114–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viswanathan M, Kahwati LC, Golin CE, et al. Medication therapy management interventions in outpatient settings: a systematic review and meta-analysis. JAMA Intern Med January 2015;175(1):76–87. [DOI] [PubMed] [Google Scholar]
- 28.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care June 2005;43(6):521–530. [DOI] [PubMed] [Google Scholar]


