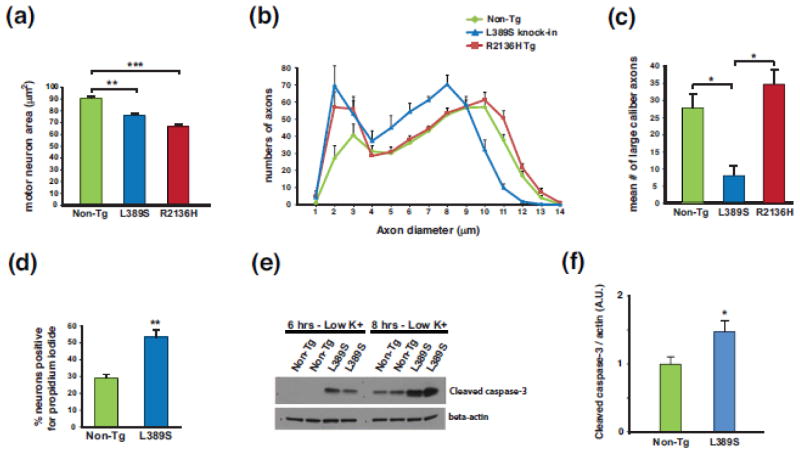
Figure 2. SETX ALS4 mice display motor neuron degeneration and neuron toxicity.
(a) We measured motor neuron area at the L5 level of the lumbar spinal cord in sections from 13 month-old mice of the indicated genotype (n = 3 mice/genotype; 100–120 motor neurons/genotype). **P < .01, ***P < .001; ANOVA with post-hoc Bonferroni test.
(b) We quantified axon numbers, measuring diameters, in the L5 motor root for mice of the indicated genotype (n = 3 mice/genotype; 800 – 850 axons counted/individual mouse).
(c) Comparison of number of large caliber (> 9 μm diameter) motor axons from (b). *P < .05; ANOVA with post-hoc Bonferroni test. Error bars = s.e.m.
(d) Quantification of propidium iodide-positive primary cortical neurons from SETX-L389S+/− mice and littermate control mice (n = 3 mice/genotype; 9 technical replicates/individual mouse). **P < .01, t-test.
(e) We cultured primary cerebellar granule neurons (CGNs) from SETX-L389S+/− mice and littermate control mice in media containing 25 mM KCl, subjected the CGNs to potassium withdrawal by switching them to 5 mM KCl for either 6 hrs or 8 hrs, and then performed immunoblot analysis of CGN protein lysates for cleaved caspase-3. Beta-actin served as the loading control.
(f) Quantification of cleaved caspase-3 level normalized to beta-actin for CGNs subjected to potassium withdrawal for 8 hrs (n = 9 mice/genotype). *P < .05, t-test.
Error bars = s.e.m.