Abstract
The objectives of this study were to elaborate an edible coating based on sodium alginate with probiotic potential and to evaluate the coating’s performance with respect to microbial viability and the characteristics of safety and freshness of minimally processed carrots stored at 8 ± 2 °C. Carrot slices were submerged in a sodium alginate solution with and without the addition of Lactobacillus acidophilus La-14 (7.36 log CFU/g), and gelling was activated by subsequent immersion in a calcium chloride solution. Physical, chemical and microbiological analyses of coated and non-coated samples were performed over a period of 19 days. At the end of this period, the viable cell count of the probiotic remained at 7.11 log CFU/g. Thus, the alginate coating was an efficient support for L. acidophilus. In addition, comparing the acidity increase between the treatments, samples with probiotic coating presented the lowest statistically significant variation, suggesting that the probiotics had reduced the metabolism of the minimally processed carrot slices. The barrier created by the coatings also contributed to the quality of the minimally processed carrots by conserving their moisture and minimizing color changes during storage. These factors are important determinants of the successful commercialization of these products.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3301-0) contains supplementary material, which is available to authorized users.
Keywords: Sodium alginate, Fresh-cut vegetables, Lactobacillus acidophilus, Viability
Introduction
A variety of studies have demonstrated that healthier living and eating habits promote good health. Due to globalization, consumers receive health-related information daily and are becoming increasingly health-conscious and demanding. This change is leading the food industry to increase the variety of foods claiming functional properties. Functional foods are those that can provide health benefits in addition to basic nutrition (Tripathi and Giri 2014), but it should be emphasized that in most cases, functional foods promote prevention of disease, rather than a cure (Saarela 2011). Among the variety of foods claiming functionality, one of the emerging classes is food containing probiotic bacteria. Probiotics are live microorganisms that promote health benefits when administered in adequate amounts (FAO/WHO 2001; Sanders 2003).
Bacteria belonging to the genera Bifidobacterium and Lactobacillus are the probiotic microorganisms most commonly employed in the food sector (Landete et al. 2016; Rigobelo 2012; Pandey et al. 2015). The species Lactobacillus acidophilus has been widely studied and attributed with characteristics, such as promoting a beneficial modulation of the metabolic activity of intestinal bacteria, which can prevent diarrhea associated with antibiotic use (Ouwehand et al. 2014); preserving intestinal integrity during radiotherapy; stimulating the immune response; and stimulating the production of lactase, which aids in the digestion of lactose and improves intestinal microflora (Demers et al. 2014). Historically, foods with probiotic claims have been derived from dairy (Prado et al. 2010), making their consumption inaccessible to those who are lactose intolerant or allergic to milk proteins (α-lactoalbumin and β-lactoglobulin). Thus, other non-dairy, raw materials have been studied as supports for probiotic organisms (Jaworska et al. 2011) with the intention of overcoming this consumption limitation.
In parallel, new habits and customs are demanding greater practicality for food. This change has contributed to the growth of the minimally processed fruit and vegetable market, with certain products undergoing physical alteration without losing their fresh produce characteristics (Alegria et al. 2010). In the field of fruit and vegetable preservation technology, edible coatings have appeared as an option to extend the shelf life of fresh produce in addition to possibly acting as probiotic culture carriers (Lacey et al. 2012; Tapia et al. 2007; Moreira et al. 2015). Sodium alginate is a polysaccharide widely employed in the elaboration of such coatings, exhibiting important characteristics in its formulations, such as resistance to gas exchanges and mechanical damage, the capacity to retard lipid oxidation in foods, and the ability to improve the flavor, texture and adhesion, protecting the cell structure of the coated vegetable (Lee and Mooney 2012).
Carrots stand out among the minimally processed vegetables, although mechanical stress and undesirable metabolic changes caused by peeling and cutting can reduce the shelf life of this vegetable compared to the in natura product, limiting the acceptability and buying intention of consumers (Fai et al. 2016).
Considering the above, the objective of the present work was to elaborate an edible coating with probiotic potential based on sodium alginate, apply it to minimally processed carrots and evaluate the shelf life of the product based on the cell viability of the L. acidophilus La-14 added to the coating and the characteristics of the safety and freshness of the carrots throughout a refrigerated storage period.
Materials and methods
Material
The freeze-dried probiotic culture L. acidophilus La-14 (Prolive-Aché, Aché, Guarulhos, Brazil) was used. The coatings were composed of sodium alginate (Dinâmica®, Diadema, Brazil), glycerol (Rioquímica, São José do Rio Preto, Brazil), sunflower oil (Cargill Agrícola S.A, Brazil) and tween 80 (Cooperativa Agroindustrial Alegrete, Brazil). Calcium chloride was used to form a gel by cross-linking with the alginate (Synth, Diadema, Brazil). Carrots (Daucus carota L., cv. Planalto) were purchased at a local market (Marília, S.P., Brazil) and were stored at 8 ± 2 °C until processing.
Minimally processed
The carrots were decontaminated by immersion in chlorinated-water (200 ppm/20 min) and sliced with a domestic cutter (Master Pro Fun Kitchen Stainless, Fun Kitchen, São José/SC, Brazil). Slices with an average thickness of 4 mm and diameters ranging from approximately 3–4 cm were immersed in the chlorinated-water solution (50 ppm/10 min), rinsed in potable water, and blotted dry to remove excess surface water (Alegria et al. 2010 with modifications). The procedure was carried out in a sanitized environment at a temperature of 20 °C.
Edible coating and addition of probiotic culture
After preliminary tests, the filmogenic solution selected for the experiments was formulated using sodium alginate (1.75 g), glycerol (0.5 g), sunflower oil (0.075 g), tween 80 (0.025 g) and water (100 g). For the coating containing probiotics, 0.67 g of a powdered, freeze-dried culture was added. The cell viability of the alginate-based solution inoculated with probiotics was 7.36 log CFU/g.
Application of the coating and storage of the minimally processed carrots
The coating was applied to the carrots using the dipping technique (Tapia et al. 2007). The 4-mm-thick carrot slices were immersed in the sodium alginate-based filmogenic solution for 2 min and later immersed in the calcium chloride solution (1.0 g/100 g water) for 1 min to promote the ionic gelation of the alginate by its reticulation with the bivalent Ca2+ ion (Tapia et al. 2007 with modifications). In sequence, 15 slices were placed on expanded polystyrene trays (0 mm × 110 mm × 30 mm) lined with acetate sheets and maintained at a temperature of 8 ± 2 °C without air circulation for 12 h to facilitate the partial dehydration of the coatings. After this period, the trays were covered and sealed with vinyl polychloride film (WYDA, Sorocaba/SP, Brazil) with a grammage of 8.71 g/m2. In addition to samples treated with the edible coating plus probiotics (T1) and without the addition of the microorganism (T2), some carrot slices were processed in parallel with no coating and stored at 8 ± 2 °C for 19 days to be used as the control (C).
Physicochemical analyses
The moisture content, titratable acidity and pH values were measured on days 0, 3, 7, 11, 15 and 19. Independent experiments for color evaluation were performed with alginate-coated slices (T2) and non-coated slices (C), and color parameters were determined on days 0, 3, 6, 9, 12, 15 and 18. The samples used to determine the moisture content, titratable acidity and pH values were ground in a domestic blender. All the analyses were done in triplicate, using 3 different trays per day.
Moisture content
The evaluation of the moisture content was started at the initial storage time (0 d) after the partial dehydration of the samples performed for 12 h at a temperature of 8 ± 2 °C. The moisture content was determined according to the gravimetric method of the AOAC official method 920.151. (2005). This measurement was performed by weighing 2 g of sample in a weighing bottle with a stopper on an analytical balance with a precision of 0.0001 (Gehaka AG 200, São Paulo/SP, Brazil) and drying in a vacuum oven (Marconi 033, Piracicaba/SP, Brazil) at 70 °C to a constant weight.
Titratable acidity and pH
Titratable acidity and pH analyses were performed at the initial storage time (0 d) after partial dehydration. The titratable acidity was determined according to the AOAC official method 942.15 (2005). The results are expressed in g of citric acid/100 g of sample. The pH values were determined using a digital pH meter, model PG1800 (Gehaka, São Paulo, Brazil), according to the AOAC official method 981.12 (2005).
Color
To verify the color of the samples with and without the edible coating (T2 and C, respectively), the ColorFlex bench spectrophotometer (HunterLab, Resto, USA) was used, and a reflectance reading of the coordinates L*, a*, and b* was obtained. The coordinate L* represents the lightness component and ranges from 0 (black) to 100 (white), while a* and b* represent the chromatic components. The coordinate a* ranges from green (− a*) to red (+ a*), and the coordinate b* ranges between blue (− b*) and yellow (+ b*). Hue-angle (°H) and chroma parameter (C*) were calculated according to Eqs. (1) and (2) listed below (Goyeneche et al. 2014). A total of 15 measurements were taken in each sample.
| 1 |
| 2 |
Microbiological analyses
Cell viability of probiotic
The probiotic viability was determined in triplicate at inoculation and after the partial dehydration (0 d) and through storage time (6, 13 and 19 d). To measure viability, 1 g of edible coating was diluted in 99 mL of 0.1% peptone water, homogenized in a previously sterilized mechanical shaker and submitted to orbital shaking at 180 rpm for 1 h at a temperature of approximately 37 °C. Serial dilutions were later prepared and deep plated in MRS agar overlaid (sealed) with agar. The plates were incubated in an incubator (BOD NI 1704, NOVA Instruments, Piracicaba/SP, Brazil) for 72 h at 37 °C (Lacey et al. 2012 with modifications).
Analysis of contaminants
The presence of contaminating microorganisms was evaluated throughout the storage period on days 0, 9 and 19, analyzing for mesophylls, yeast, mold, total and thermotolerant coliforms, coagulase-positive staphylococci and Bacillus cereus, according to the methods of the American Public Health Association (Downes and Ito 2001; Lancette and Bennett 2001; Morton 2001). The presence or absence of Salmonella spp. was also determined using the traditional identification method described by the International Organization for Standardization 6579:2007 (ISO 2002).
Statistical analysis
The results obtained were submitted to an analysis of variance (ANOVA) followed by Tukey’s test, using the BIOESTAT 5.0 program (Ayres et al. 2007). Differences were considered significant to be with a p value of < 0.05.
Results
Moisture content
Previously of the minimally processed, the mean moisture content of in natura carrot was approximately 90.5%, lower than the mean values of the carrot slices with coatings, since the coating solution had a moisture content approximately 97.5%. Table 1 shows the moisture contents of the packaged samples throughout the storage period for the three treatments from the initial storage day (0 d).
Table 1.
Mean values for the moisture content (%) of the minimally processed carrot slices with edible coating and added probiotic (T1), with edible coating (T2) and without edible coating (C) during 19 days of storage at 8 ± 2 °C
| Treat | Storage (days) | |||||
|---|---|---|---|---|---|---|
| 0 (M ± SD) |
3 (M ± SD) |
7 (M ± SD) |
11 (M ± SD) |
15 (M ± SD) |
19 (M ± SD) |
|
| T1 | 91.8 ± 0.1b | 92.2 ± 0.2b | 91.7 ± 0.1ª | 91.4 ± 0.0ª | 91.0 ± 0.0b | 90.5 ± 0.0b |
| T2 | 93.1 ± 0.2a | 92.9 ± 0.1ª | 91.2 ± 0.1b | 91.3 ± 0.0ª | 91.4 ± 0.0a | 91.4 ± 0.0a |
| C | 89.8 ± 0.1c | 89.4 ± 0.1c | 89.9 ± 0.1c | 89.4 ± 0.0b | 89.5 ± 0.0c | 89.6 ± 0.1c |
M mean of triplicate values, SD standard deviation
Means followed by the same letter in the same column are not significantly different according to Tukey’s test
The results obtained in terms of moisture content suggested that the control sample (C) without the protection of a coating showed a greater level of dehydration. The sample’s appearance was also compromised by white discoloration after 19 days of storage, as seen in figure of the supplementary material. It can be concluded that treatments T1 and T2 protected the minimally processed carrot slices from whitening throughout the storage period.
Titratable acidity and pH
Table 2 presents the mean values obtained from the three treatments for titratable acidity and pH during the 19 days of storage. The results show a slight reduction in pH for all of the treatments. At the end of the 19 days, the decreases were 0.21, 0.13 and 0.22 for T1, T2 and C, respectively.
Table 2.
Mean values for titratable acidity (% citric acid) and pH of minimally processed carrot slices with edible coating with added probiotic (T1), with edible coating (T2) and without edible coating (C) during 19 days of storage at 8 ± 2 °C
| Storage (days) | Titratable acidity | pH | ||||
|---|---|---|---|---|---|---|
| T1 (M ± SD) |
T2 (M ± SD) |
C (M ± SD) |
T1 (M ± SD) |
T2 (M ± SD) |
C (M ± SD) |
|
| 0 | 0.58 ± 0.00b | 0.58 ± 0.00b | 0.77 ± 0.01a | 6.60 ± 0.04b | 6.45 ± 0.02c | 6.72 ± 0.03a |
| 3 | 0.78 ± 0.00a | 0.77 ± 0.02a | 0.84 ± 0.12a | 6.69 ± 0.03a | 6.39 ± 0.05b | 6.39 ± 0.03b |
| 7 | 0.84 ± 0.10b | 0.95 ± 0.14b | 1.17 ± 0.01a | 6.27 ± 0.05b | 6.40 ± 0.03a | 6.50 ± 0.06a |
| 11 | 0.96 ± 0.01b | 1.03 ± 0.11ab | 1.17 ± 0.00a | 6.48 ± 0.03b | 6.52 ± 0.02b | 6.65 ± 0.04a |
| 15 | 0.87 ± 0.00c | 1.00 ± 0.06b | 1.15 ± 0.01a | 6.43 ± 0.03b | 6.42 ± 0.02b | 6.57 ± 0.02a |
| 19 | 0.77 ± 0.00c | 0.97 ± 0.00b | 1.13 ± 0.02a | 6.39 ± 0.03b | 6.32 ± 0.02c | 6.50 ± 0.01a |
M mean of triplicate values, SD standard deviation
Means followed by the same letter in the same line are not significantly different according to Tukey’s test
Table 2 shows the results obtained for titratable acidity. Samples T1 and T2 showed lower values than the control on the first day due to interference from the pH of the sodium alginate solution (pH ≅ 7.0). Sample T1, with the edible coating and added probiotic, showed numerically lower values for acidity throughout the whole storage period.
Color
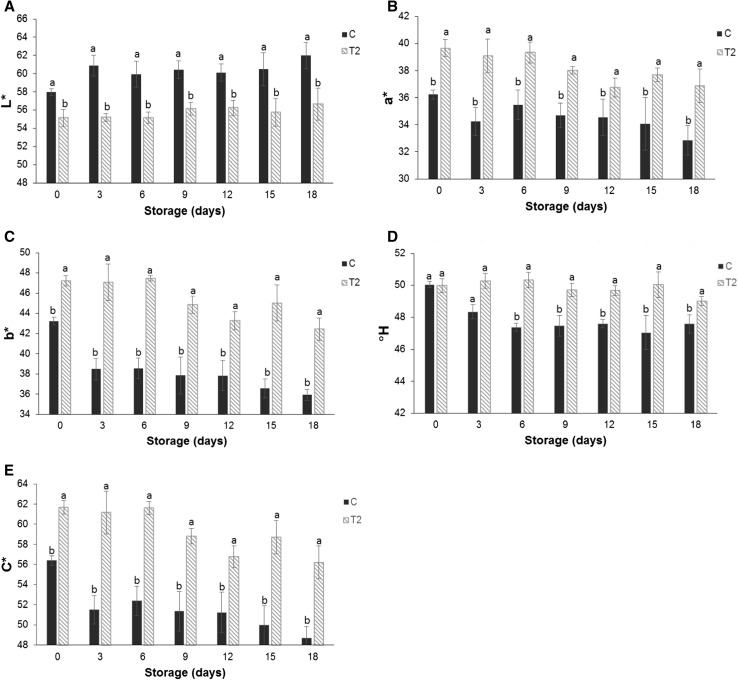
In the present research, the chromatic parameters (L*, a* and b*) (Fig. 1a–c) of the sample with the edible coating (T2) presented smaller variations compared to sample C throughout the storage period. The two samples showed a slight tendency towards decreasing intensity in the red (a*) and yellow (b*) colors and an increase in lightness (L*).
Fig. 1.
Effect of the edible coating application on the specific activity of the color parameters: a L*, b a*, c b*, d °H and e color saturation (C*) of the minimally processed carrot (C) with edible coating (T1). Each value represents a mean of three independent replicates. Vertical bars represent ± SD of means
The color parameter indicators of quality (°H and C*) (Fig. 1d, e) also showed a behavior similar to the chromatic parameters, i.e., decreasing values throughout the storage period, except the °H angle of the coated samples, which did not vary during the storage period.
Viability of the Lactobacillus acidophilus
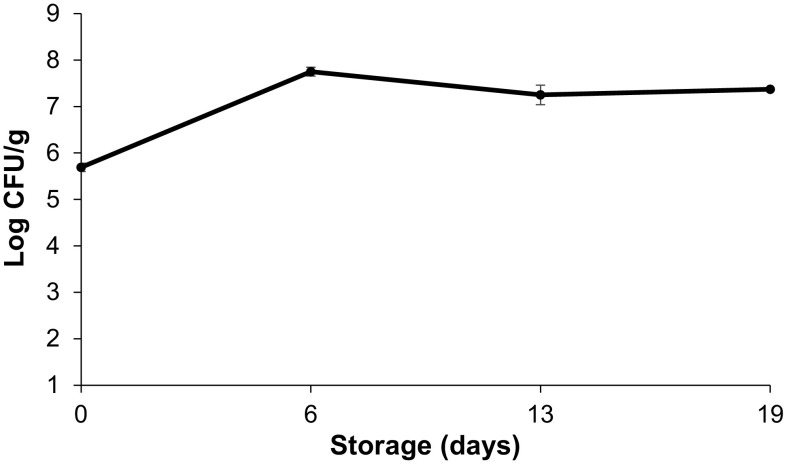
Lactobacillus acidophilus was inoculated at a concentration of 7.36 log CFU/g in coating solution. Figure 2 shows the results obtained for the cell viability of the L. acidophilus in the coating of the minimally processed carrot slices throughout storage. There was a decrease in the viability of the microorganism after coating dehydration, i.e., at 1st determination, but it recovered during the rest of the storage period.
Fig. 2.
Log CFU of Lactobacillus acidophilus/g in the alginate coating of minimally processed carrot slices as a function of storage time. Vertical bars represent ± SD of means
Microbial contaminants
Table 3 shows the results of the aerobic mesophylls and yeast and mold analyses carried out to evaluate the microbiological quality of the products during storage.
Table 3.
Microbiological quality of minimally processed carrot slices with alginate coating with added Lactobacillus acidophilus (T1), with alginate coating (T2) and without alginate coating (C) at different storage times at 8 ± 2 °C
| Treat | Microorganism | Storage (days) | ||
|---|---|---|---|---|
| 0 | 9 | 19 | ||
| T1 | Aerobic mesophylls | abs | 3.0 × 105 CFU/g | 4.5 × 105 CFU/g |
| Yeasts and molds | abs | abs | abs | |
| T2 | Aerobic mesophylls | abs | 3.0 × 105 CFU/g | 3.0 × 105 CFU/g |
| Yeasts and molds | abs | 1.0 × 102 CFU/g | 2.0 × 102 CFU/g | |
| C | Aerobic mesophylls | abs | 3.0 × 105 CFU/g | 3.0 × 105 CFU/g |
| Yeasts and molds | abs | abs | abs | |
abs absent
The samples analyzed showed coliforms at 45 °C and salmonella below the limits determined by Resolution RDC no. 12 of January, 2001 (Brasil 2001). They also showed an absence of total coliforms, coagulase-positive staphylococci and Bacillus cereus, indicating that they were safe for consumption.
In the present study, the maximum number of aerobic mesophylls was 4.5 × 105 CFU/g, and the maximum yeast and mold count was 2 × 102 CFU/g, showing that the 3 varieties of carrot samples analyzed were not at critical levels that could lead to sensory changes of the product during the 19 days of storage. Only T2 showed the presence of yeast and mold after 9 days of storage.
Discussion
Moisture content
As shown in Table 1, the highest moisture content throughout the storage period was observed in the coated samples (T1 and T2), since the coating aggregated more water to the samples compared to the non-coated carrots. The coated samples lost more than 1% of their initial moisture during storage. However, at the end of the storage period, their moisture content was still higher than that of the control samples, demonstrating that polysaccharide coatings can slow down moisture loss from food products (Bourtoom 2008). Ahmed and Butt (2014) observed the same behavior when fresh strawberries, both non-coated and coated with a sodium alginate solution (2.0, 2.5 and 3.0%), were stored for 20 days in controlled climate chamber (4 ± 1 °C, 85% RH).
With respect to the appearance of the minimally processed carrot slices, dehydration and structural alterations of the surface cell layers are the main causes of whitening and loss of orange color (Leceta et al. 2015; Lavelli et al. 2006).
Titratable acidity and pH
It could be inferred that the variations in acidity observed in the present study were due to the metabolic processes of the vegetable and the deteriorative microorganisms developing during storage, resulting in an increased concentration of organic acids. Oliveira et al. (2014), working with cubes of melon immersed in a citric acid: sodium citrate (1:1) solution containing Lactobacillus rhamnosus, related the increase in acidity during storage at temperatures of 6 and 15 °C for a maximum of 120 h to a degradation of the fruit sugars and consequent liberation of acids. As seen in Table 2, the coating containing the probiotic (T1) partially inhibited the increase in acidity of the minimally processed carrot slices. The coating without probiotic (T2) also contributed to a reduction in acids compared to the control, although to a lesser extent.
Color
The surface color of carrots is a property that determines consumer acceptance (Leceta et al. 2015). Peeling and cutting cause chemical and biochemical changes in minimally processed carrots, which affect their shelf life and appearance, especially their color.
The presence of a sodium alginate-based edible coating demonstrated improved color parameters during the storage period of the product (Fig. 1a, b). In T2, there was a significant increase (p < 0.05) of 12.5 and 18.1% in the values of color parameters a* and b*, respectively, when compared to the control (C) on the 18th day of storage. The initial difference of the color parameters between samples C and T2 is also due to the presence of the coating, which has a slightly yellowish color (Leceta et al. 2015). However, the difference tends to be accentuated as the storage time goes, especially in regard to the parameter b*, because its reduction in the control samples occurs at a rate higher than in the coated samples.
At the same time, although the coating slightly reduced the lightness of the carrots, an increase in L* values in the C samples was observed over the storage time, and it was a greater increase than in the coated samples, indicating a gradual appearance of a faded-orange color on the surface of the carrot (Fig. 1a). The dehydration on the surface of the minimally processed carrot might have caused the white blush of the sample, which also presented an opaque appearance due to the loss of the vivid orange color. This was evidenced by the low C* values (Fig. 1e) throughout storage. As the chroma decreased, the color became less intense and, along with the increase in L*, provided a whitish appearance to the minimally processed carrot (C).
The orange tonality was not altered by the presence of the coating, as observed on the first day, when the C and T2 samples presented the same value of °H. However, over the storage time, there was very little change in orange tonality in the coated samples, whereas the control samples exhibited a slightly more pronounced orange color, nearly red.
Pushkala et al. (2012) correlated the decreased moisture and loss of mass with the surface dehydration of the control sample (shredded carrot) and the consequent reduction in orange color of the product. By protecting the surface from dehydration, the edible coating reduced the weight loss compared to the control sample and maintained greater surface hydration in addition to smaller variations of L*. The hydrophilic substances that constituted the coating might have helped to maintain greater humidity in the surface of the plant tissue, thus avoiding dehydration and consequent discoloration, as noted by Vargas et al. (2008).
Viability of the Lactobacillus acidophilus
The decreased viability of the L. acidophilus at the start of the experiment likely occurred due to its adaptation to the coating to which it was added. However, the cell viability was duly recovered (above 107 CFU/g of coating) during the storage period (Fig. 2), indicating that the alginate-based coating used for the minimally processed carrot was an adequate vehicle for the probiotic bacteria studied. One gram of the coating was sufficient to cover approximately three carrot slices. With respect to the probiotic count in the food, a concentration of between 106 and 107 CFU/g is recommended at the moment of consumption (Cruz et al. 2009). According to these values, in order for the carrot treated with the coating containing L. acidophilus to present probiotic potential, a minimal daily consumption of 77 g of the product is required.
Tapia et al. (2007), working with papaya coated with a 2% sodium alginate coating with added Bifidobacterium lactis Bb-12, obtained results for cell viability similar to those found in the present study. Angiolillo et al. (2014), who obtained cell viability values for Lactobacillus rhamnosus to the order of 4.52 × 107 CFU/g for cheese coated with sodium alginate, also obtained similar results.
The inclusion of L. acidophilus in the alginate-based coating probably contributed to the cell stability of the bacterium. The coating technique allowed the vegetable to present probiotic potential without causing any significant physical or chemical alterations.
Microbial contaminants
In the present study, there was an absence of potentially pathogenic bacteria in the samples, demonstrating that the preventative measures taken, involving sanitizing and low temperature storage, contributed to the maintenance of microbiological quality. At the end of the 19 days of storage, all of the samples submitted to all of the treatments were fit for consumption.
Aerobic mesophylls and fungi were present in acceptable amounts according to the literature. For carrots cut in the form of sticks to be accepted, the maximum aerobic mesophyll bacterial count should not exceed 5 × 108 CFU/g (Lavelli et al. 2006).
T2 demonstrated presence of yeast and mold, which was likely due to the high moisture content of the coating and excessive handling during its application, since sample C (control) showed no such growth. On the other hand, sample T1, which contained lactic bacteria in addition to the same coating, although with a slightly lower water content due to the addition of the freeze dried microorganism, also showed no yeast or mold growth. It is possible that the presence of L. acidophilus in sample T1 inhibited fungal growth during the 19 days of storage, since sample T2 (coating without probiotic) showed contamination by these deteriorative organisms in the analysis of the last 2 days of storage. Moreover, the acidity was lower in the sample containing the probiotics, suggesting that the probiotic microorganisms could have contributed to the preservation of the carrot slices. This could be due to the fact that L. acidophilus species are involved in the production of anti-microbial substances that act against other bacteria, viruses, protozoa and fungi (Stiles and Hastings 1991; Zhao et al. 2015). Angiolillo et al. (2014) demonstrated that the probiotic Lactobacillus rhamnosus could also have an antimicrobial effect since, when added to a sodium alginate coating applied to cheese, it exerted a slight inhibition against Pseudomonas spp. and bacteria of the family Enterobacteriaceae. Thus, microorganisms can also contribute to increasing the safety of the food to which they are added in addition to extending its shelf life, although more research is required to confirm these effects.
Conclusion
The alginate-based edible coating used to coat the minimally processed carrot slices was efficient as a support for freeze-dried L. acidophilus, thereby conserving its cell viability. This indicates the possible probiotic potential of a food product of vegetable, and not animal, origin.
The use of these coatings (with and without probiotics) applied to minimally processed carrot slices presented advantages, such as greater preservation of moisture content and color, which are determining factors for the commercialization of these products. In addition, it was shown that the whitish surface discoloration was minimized when the coatings were used. The presence of L. acidophilus in the coating likely reduced the metabolism of the minimally processed carrot slices, resulting in a lower variation in acidity during storage. There are also indications that the probiotic can inhibit fungal contamination of the product, although more research is required to confirm these effects.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (Project No. 2014/11514-8).
References
- Ahmed W, Butt MS. Preserving strawberry (Fragaria Ananasa) using alginate and soy based edible coatings. Am J Food Sci Technol. 2014;2:158–161. doi: 10.12691/ajfst-2-5-4. [DOI] [Google Scholar]
- Alegria C, Pinheiro J, Gonçalves EM, Fernandes I, Moldão M, Abreu M. Evaluation of a pre-cut heat treatment as an alternative to chlorine in minimally processed shredded carrot. Innov Food Sci Emerg Technol. 2010;11:155–161. doi: 10.1016/j.ifset.2009.10.008. [DOI] [Google Scholar]
- Angiolillo L, Conte A, Faccia M, Zambrini AV, Del Nobile MA. A new method to produce synbiotic Fiordilatte cheese. Innov Food Sci Emerg Technol. 2014;22:180–187. doi: 10.1016/j.ifset.2013.09.010. [DOI] [Google Scholar]
- A.O.A.C. Official methods of analysis of A.O.A.C international. 18. Arlington: Association of Official Analytical Chemists; 2005. [Google Scholar]
- Ayres M, JrM A, Ayres DL, Santos AAS. BioEstat: aplicações estatísticas nas áreas das ciências biológicas e médicas. Belém: Sociedade Civil Mamirauá, MCT-CNPq; 2007. [Google Scholar]
- Bourtoom T. Edible films and coatings: characteristics and properties. Int Food Res J. 2008;15:237–248. [Google Scholar]
- Brasil. Agência Nacional de Vigilância Sanitária . Resolução RDC nº 12, de 02 de janeiro de 2001. Aprova o Regulamento Técnico sobre padrões microbiológicos para alimentos. Brasília: Diário Oficial da União; 2001. [Google Scholar]
- Cruz AG, Antunes AEC, Sousa ALOP, Faria JAF, Saad SMI. Ice cream as a probiotic food carrier. Food Res Int. 2009;42:1233–1239. doi: 10.1016/j.foodres.2009.03.020. [DOI] [Google Scholar]
- Demers M, Dagnault A, Desjardin J. A randomized double-blind controlled trial: impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin Nutr. 2014;33:761–767. doi: 10.1016/j.clnu.2013.10.015. [DOI] [PubMed] [Google Scholar]
- Downes FP, Ito K, editors. Compendium of methods for the microbiological examination of foods. 4. Washington: American Public Health Association (APHA); 2001. [Google Scholar]
- Fai AEC, Souza MRA, Barros ST, Bruno NV, Ferreira MSL, Gonçalves ECBA. Development and evaluation of biodegradable films and coatings obtained from fruit and vegetable residues applied to fresh-cut carrot (Daucus carota L.) Postharvest Biol Technol. 2016;112:194–204. doi: 10.1016/j.postharvbio.2015.09.021. [DOI] [Google Scholar]
- FAO/WHO . Evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Córdoba: Report of a Joint Food and Agriculture Organization of the United Nations, World Health Organization Expert Consultation; 2001. [Google Scholar]
- Goyeneche R, Agüero MV, Roura S, Di Scala K. Application of citric acid and mild heat shock to minimally processed sliced radish: color evaluation. Postharvest Biol Technol. 2014;93:106–113. doi: 10.1016/j.postharvbio.2014.02.011. [DOI] [Google Scholar]
- Tripathi MS, Giri SK. Probiotic functional foods: survival of probiotics during processing and storage. J Funct Foods. 2014;9:225–241. doi: 10.1016/j.jff.2014.04.030. [DOI] [Google Scholar]
- ISO 6579 (2002) Microbiology of food and animal feeding stuffs: horizontal method for the detection of Salmonella spp., 4th edn. The International Organization for Standardization, amendment 1, 15 July 2007
- Jaworska D, Neffe K, Kolozyn-Krajewska D, Dolatowski ZJ. Survival during storage and sensory effect of potential probiotic lactic acid bacteria Lactobacillus acidophilus Bauer and Lactobacillus casei Bif3′/IV in dry fermented pork loins. Int J Food Sci Technol. 2011;46:2491–2497. doi: 10.1111/j.1365-2621.2011.02772.x. [DOI] [Google Scholar]
- Lacey AML, López-Caballero ME, Gómez-Estaca J, Gómez-Guillén MC, Montero P. Functionality of Lactobacillus acidophilus and Bifidobacterium bifidum incorporated to edible coatings and films. Innov Food Sci Emerg Technol. 2012;16:277–282. doi: 10.1016/j.ifset.2012.07.001. [DOI] [Google Scholar]
- Lancette GA, Bennett RW. Staphylococcus aureus and staphylococcal enterotoxins. In: Downes FP, Ito K, editors. Compendium of methods for the microbiological examination of foods, Chapter 39. 4. Washington: American Public Health Association; 2001. pp. 387–403. [Google Scholar]
- Landete JM, Medina M, Arque JL. Fluorescent reporter systems for tracking probiotic lactic acid bacteria and bifidobacteria. World J Microbiol Biotechnol. 2016;32:119. doi: 10.1007/s11274-016-2077-5. [DOI] [PubMed] [Google Scholar]
- Lavelli V, Pagliarini E, Ambrosoli R, Minati JL, Zanoni B. Physicochemical, microbial, and sensory parameters as indices to evaluate the quality of minimally-processed carrots. Postharvest Biol Tec. 2006;40:34–40. doi: 10.1016/j.postharvbio.2005.12.004. [DOI] [Google Scholar]
- Leceta I, Molinaro S, Guerrero P, Kerry JP, Caba K. Quality attributes of map packaged ready-to-eat baby carrots by using chitosan-based coatings. Postharvest Biol Technol. 2015;100:142–150. doi: 10.1016/j.postharvbio.2014.09.022. [DOI] [Google Scholar]
- Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira MR, Cassani L, Martín-Belloso O, Soliva-Fortuny R. Effects of polysaccharide-based edible coatings enriched with dietary fiber on quality attributes of fresh-cut apples. J Food Sci Technol. 2015;52(12):7795–7805. doi: 10.1007/s13197-015-1907-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton RD. Aerobic plate count. In: Downes FP, Ito K, editors. Compendium of methods for the microbiological examination of foods, Chapter 7. 4. Washington: American Public Health Association; 2001. pp. 63–67. [Google Scholar]
- Oliveira PM, Leite BRC, Jr, Martins ML, Martins EMF, Ramos AM. Minimally processed yellow melon enriched with probiotic bactéria. Semin Ciênc Agrár. 2014;35:2415–2426. doi: 10.5433/1679-0359.2014v35n5p2415. [DOI] [Google Scholar]
- Ouwehand AC, Donglian C, Weijian X, Steart M, Nid J, Stewart T, Miller LE. Probiotics reduce symptoms of antibiotic use in a hospital setting: a randomized dose response study. Vaccine. 2014;32:458–463. doi: 10.1016/j.vaccine.2013.11.053. [DOI] [PubMed] [Google Scholar]
- Pandey KR, Naik SR, Vakil BV. Probiotics, prebiotics and synbiotics: a review. J Food Sci Technol. 2015;52(12):7577–7587. doi: 10.1007/s13197-015-1921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado S, Romalde JL, Braja JL. Review of probiotics for use in bivalve hatcheries. Vet Microbiol. 2010;145:1–11. doi: 10.1016/j.vetmic.2010.08.021. [DOI] [PubMed] [Google Scholar]
- Pushkala R, Parvathy KR, Srividya N. Chitosan powder coating, a novel simple technique for enhancement of shelf life quality of carrot shreds stored in macro perforated LDPE packs. Innov Food Sci Emerg Technol. 2012;16:11–20. doi: 10.1016/j.ifset.2012.03.003. [DOI] [Google Scholar]
- Rigobelo EC. Probiotics. Rijeka: InTeck; 2012. pp. 44–46. [Google Scholar]
- Saarela M. Functional foods: concept to product. Woodhead Publishing series in food science, technology and nutrition. 2. Cambridge: Woodhead Publishing; 2011. [Google Scholar]
- Sanders ME. Probiotics: considerations for human health. Nutr Rev. 2003;61:91–99. doi: 10.1301/nr.2003.marr.91-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles ME, Hastings JW. Bacteriocin production by lactic acid bacteria: potential for use in meat preservation. Trend Food Sci Technol. 1991;2:247–251. doi: 10.1016/0924-2244(91)90706-O. [DOI] [Google Scholar]
- Tapia MS, Rojas-Grau MA, Rodríguez FJ, Ramírez J, Carmona A, Martin-Belloso O. Alginate- and Gellan-based edible films for probiotic coatings on fresh-cut fruits. J Food Sci. 2007;2:190–196. doi: 10.1111/j.1750-3841.2007.00318.x. [DOI] [PubMed] [Google Scholar]
- Vargas M, Pastor C, Chiralt A, McClements DJ, González-Martinez C. Recent advances in edible coatings for fresh and minimally processed fruits. Crit Rev Food Sci Nutr. 2008;48:496–511. doi: 10.1080/10408390701537344. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Jiang L, Liu T, Wang M, Cao W, Bao Y, Qin J. Construction and immunogenicity of the recombinant Lactobacillus acidophilus pMG36e-E0-LA-5 of bovine viral diarrhea vírus. J Virol Methods. 2015;225:70–75. doi: 10.1016/j.jviromet.2015.09.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.