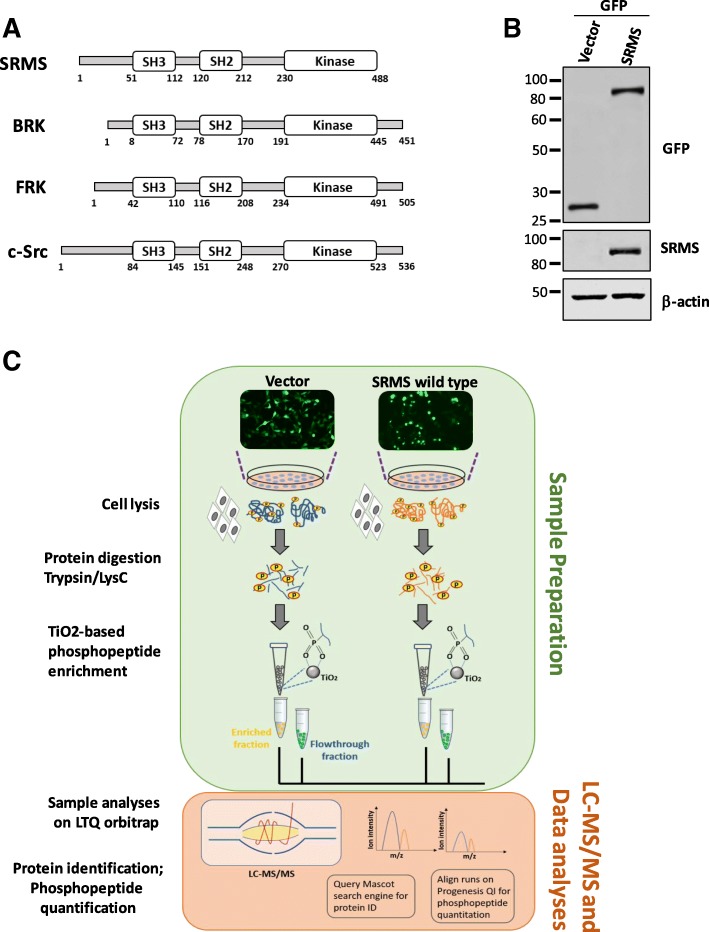
Fig. 1.
Phosphoproteomics analyses of cells expressing ectopic wild-type SRMS. a Schematic representation of the domain structure of SRMS, BRK and FRK (BRK family kinases), and c-Src, depicting the SH3, SH2 and kinase domains. The amino acid numbers indicate the length of the domains and the full-length protein. b Immunoblotting analyses was performed on a portion of the lysates derived from HEK293 cells expressing either the empty vector (GFP alone) or vector expressing GFP-SRMS (wild-type) and used for subsequent phosphopeptide enrichment analysis. The lysates were probed with antibodies against GFP and SRMS. Immunoblotting with antibodies against β-actin was used to assess the loading of total proteins. c Schematic representation of the label-free quantitation-based phosphoproteomics workflow using cells expressing GFP alone (the empty vector control) or cells expressing GFP-SRMS wild type. The cells were lysed in RIPA buffer followed by dual enzymatic digestion (Trypsin/Lys-C) and phosphopeptide enrichment using TiO2 resin. Both, enriched and flowthrough fractions were analysed by LC-MS/MS and data analyses performed using the MASCOT search engine (for protein identification) and PROGENESIS QI tool (for phosphopeptide quantitation)