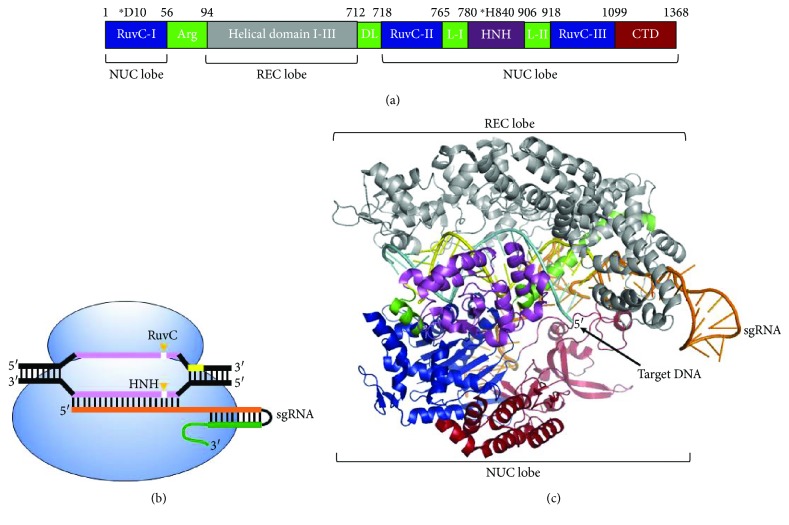
Figure 1.
Overall organization, structure, and function of CRISPR-associated protein 9 (Cas9) from Streptococcus pyogenes (SpCas9). (a) Schematic representation of the domain organization of the SpCas9. Asterisks denote catalytical residues. (b) Cas9 (blue) requires a sgRNA that has a 20 bp region complementary to the target DNA. Cas9 requires two RNA components—CRISPR RNA (crRNA; orange) and transactivating RNA (tracrRNA; green). sgRNA is a chimeric RNA in which crRNA and tracrRNA are fused through a linker. PAM sequence (5′-NGG-3′) is shown in yellow and is crucial for binding and cleavage. DNA cleavage occurs in two different domains: the HNH domain that cuts the target strand and RuvC domain that cleaves the nontarget strand. (c) Cartoon representation of the crystal structure of SpCas9 (PDB 4UN3). Cas9 domains are colored according to the scheme in (a). Abbreviations: Arg: arginine-rich bridge helix; DL: disordered linker; CTD: C-terminal domain; NUC: nuclease lobe; PAM: protospacer-adjacent motif; REC: recognition lobe.