Figure 3.
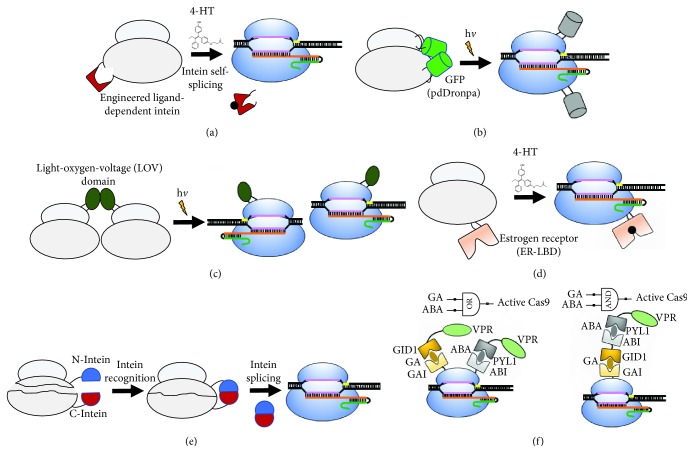
Selected case studies of engineering dCas9 by domain fusion. (a) Intein insertion inactivates dCas9, and in the presence of 4-HT, the inserted intein (red) undergoes self-splicing and restores the active Cas9 structure and function [70]. (b) Insertion of an engineered GFP (pdDronpa) that dimerizes in the dark and prevents DNA binding. On light illumination, pdDronpa dissociates and enables the binding of dCas9 to DNA [71]. (c) In the absence of light, the LOV domain (dark green) maintains a stable dimer that sterically blocks dCas9 function. Light induces LOV dissociation and Cas9 activation [74]. (d) Domain insertion of a ligand-binding domain (LBD) of the estrogen receptor ER leads to an allosteric activation by 4-HT [75]. Rather than domain insertion, an end-to-end fusion with ER-LBD results to nuclear translocation by 4-HT [58]. (e) A split Cas9 composed of two separate fragments is fused to intein sequences that perform self-splicing upon dimerization, leading to fully active Cas9 [83]. (f) The dCas9 fusion with multiple domains can generate a multi-input system and produce logic gates [57]. A VPR–SpdCas9 construct induces gene expression in the presence of gibberellin (GA) OR/AND abscisic acid (ABA). A light gray color of the Cas9 indicates that the protein is inactive or less active while blue represents the active state.