Figure 1. Canonical insulin signalling pathways.
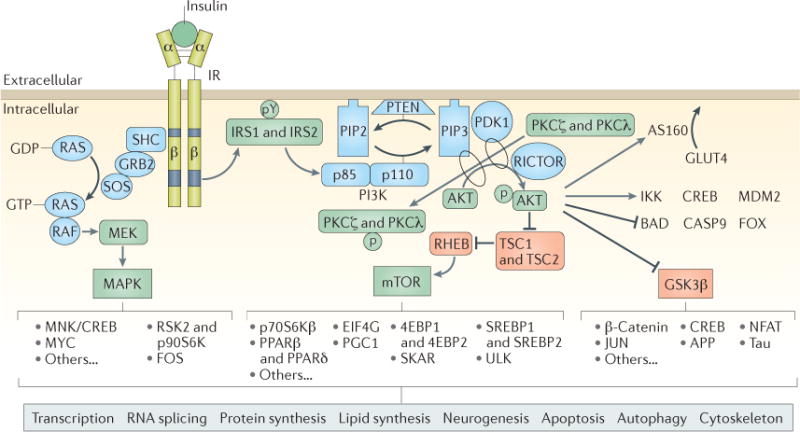
Insulin binds extracellular α-subunits of the insulin receptor (IR), leading to dimerization and autophosphorylation of β-subunits and activation of its kinase activity. The IR phosphorylates select tyrosine residues (pY) on insulin receptor substrate 1 (IRS1) and IRS2, leading to exposure of binding sites for signalling partners. IRS1 and IRS2 recruit and activate the phosphoinositide 3-kinase (PI3K) complex, which then phosphorylates and activates AKT, the major node of the insulin signalling cascade, as well as protein kinase Cζ (PKCζ) and PKCλ. Activated AKT has many downstream effects: of greatest relevance to systemic glucose control, AKT phosphorylates AKT substrate of 160 kDa (AS160; also known as TBC1D4), which controls the translocation of glucose transporter type 4 (GLUT4) to the cell membrane for uptake of glucose into muscle, adipose and some neurons. AKT-mediated activation of mTOR and the downstream targets of mTOR serves to regulate protein and lipid synthesis and many aspects of cell metabolism, growth, survival and autophagy. Phosphorylation of glycogen synthase kinase 3β (GSK3β) by AKT inhibits the constitutive activity of this key kinase. GSK3β has many protein substrates, such as glycogen synthase, β-catenin, microtubule-associated proteins (including tau), intermediate filaments, cAMP-responsive element-binding protein (CREB) and others. Through these diverse proteins, insulin and GSK3β signalling play important parts in the regulation of cellular proliferation, migration, glucose regulation, apoptosis and neuroplasticity. AKT kinase activity also directly activates proteins such as inhibitor of nuclear factor-κB kinase (IKK), CREB and E3 ubiquitin-protein ligase Mdm2 (MDM2) to regulate transcription, cytokine production and cell survival, and it directly inhibits selected proteins, including regulators of apoptosis (Bcl2-associated agonist of cell death (BAD) and caspase 9 (CASP9)) and Forkhead box protein (FOX) transcription factors. Independent of IRS1 and IRS2 and AKT, IR kinase activity initiates the activation of the mitogen-activated protein kinase (MAPK) pathway, which is especially important for regulating the transcription of CREB, Myc proto-oncogene protein (MYC) and ribosomal protein S6 kinase 2 (RSK2; also known as S6Kα3), affecting cell proliferation, differentiation, innate and adaptive immune function and neuroplasticity. Importantly, AKT, GSK3β, mTOR and MAPK themselves provide feedback autoregulation of IRS1 and IRS2, inhibiting their activity through site-specific serine phosphorylation. 4EBP, eukaryotic translation initiation factor 4E binding protein; APP, amyloid precursor protein; EIF4G, eukaryotic translation initiation factor 4γ; FOS, proto-oncogene c-Fos; GRB2, growth factor receptor-bound protein 2; JUN, transcription factor AP-1; MEK, MAPK/ERK kinase (also known as MAPKK); MNK, MAP kinase signal-interacting kinase (also known as MKNK); NFAT, nuclear factor of activated T cells; p70S6Kβ, p70 ribosomal S6 kinase β (also known as S6Kβ2); p90S6K, 90 kDa ribosomal protein S6 kinase 1 (also known as S6Kα1); PDK1, 3-phophoinositide-dependent protein kinase 1; PGC1, PPARγ coactivator 1; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; PPAR, peroxisome proliferator-activated receptor; RICTOR, rapamycin-insensitive companion of mTOR; SHC, SHC-transforming protein; SKAR, S6K1 Aly/REF-like target (also known as POLDIP3); SOS, son of sevenless homologue; SREBP, sterol regulatory element-binding protein; TSC1, hamartin; TSC2, tuberin.
