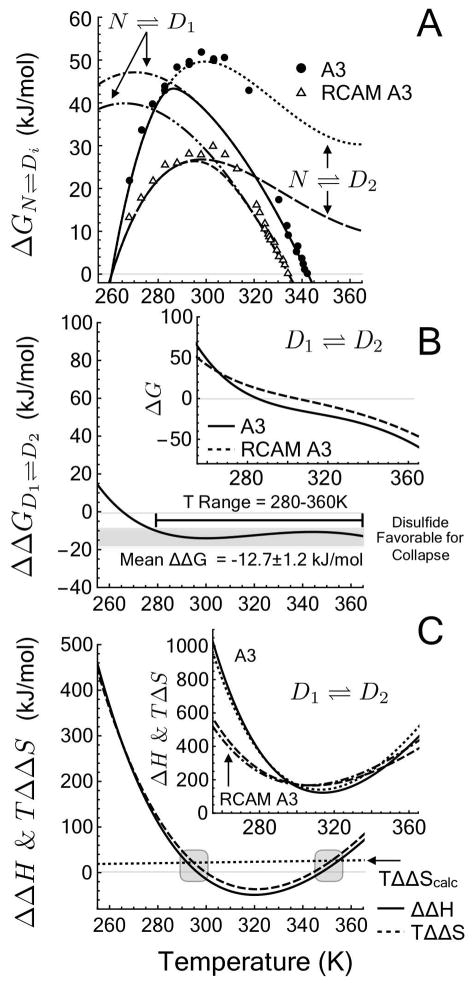
Figure 8.
Thermodynamic contribution of a disulfide bond in the absence of urea. A) ΔG. Unfolding free energy calculated from Linear Extrapolation Method (data points) and derived from the 3–state model of denaturation from N ⇌ D1 and N ⇌ D2. B) ΔΔGD1⇌D2. Free energy of a crosslinking the denatured state with a disulfide bond. Inset: ΔGD1⇌D2. Free energy of denatured state collapse from D1 to D2. C) ΔΔHD1⇌D2 and TΔΔSD1⇌D2. Enthalpic and entropic contributions to the free energy of crosslinking the denatured state and TΔΔScalc calculated from polymer theory, Eq. 16 (Gray area represents the predicted temperature range where ΔΔH = 0) [27]. Inset: ΔHD1⇌D2 and TΔSD1⇌D2. Enthalpic and entropic contributions to the free energy of denatured state collapse from D1 to D2.