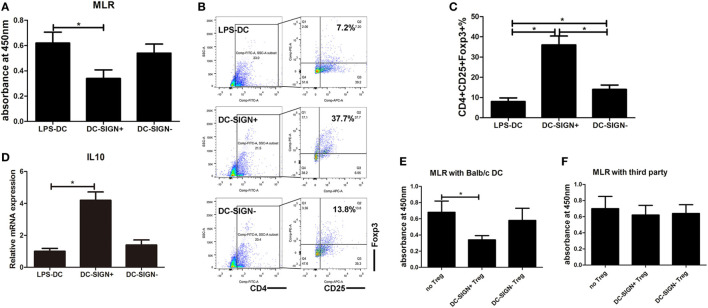
Figure 4.
DC-SIGN+ subsets induced by enforced MALAT1 exert a more potent tolerogenic ability. After pMALAT1 transfection and LPS stimulation, dendritic cells (DCs) were sorted by MACS into DC-SIGN+ DCs and DC-SIGN− DCs. Then, these DCs were conditioned with mitomycin C (10 mg/ml, 2 h) and cocultured with allogeneic T cells for 48 h. (A) T cell proliferation initiated by DCs was assessed by BrdU-ELISA. DC-SIGN+ DCs exhibited significantly more suppressive effects on primed T cell responses than did LPS-stimulated DCs and DC-SIGN− DCs. (B,C) The numbers of Tregs (CD4+CD25+Foxp3+) in T cell cocultures were assessed by flow cytometry (B) and are shown as percentages (C). DC-SIGN+ subsets, but not DC-SIGN− subsets, induced the generation of Tregs compared with both LPS-DCs and DC-SIGN− DCs. (D) IL10 mRNA expression levels in these isolated Tregs were assessed by quantitative real-time reverse transcription PCR. IL10 mRNA levels in Tregs induced by DC-SIGN+ populations were significantly higher than those in Tregs induced by LPS-DCs or DC-SIGN− populations. (E,F) Tregs (CD4+CD25+) isolated from different DC and T cell cocultures by MACS were added to a new coculture of mitomycin C-conditioned DCs (syngeneic or third party) and T cells (Treg:T cell:DC ratio of 10:10:1). The suppressive functions of Tregs on DC-primed T cell responses were assessed by BrdU-ELISA. The DC-SIGN+ subpopulation-induced Tregs, but not those induced by DC-SIGN− subsets, showed marked inhibition of DC-primed T cell proliferation. The data are presented as the mean ± SD from at least three independent experiments. *P < 0.05; **P < 0.01.