Scheme 1.
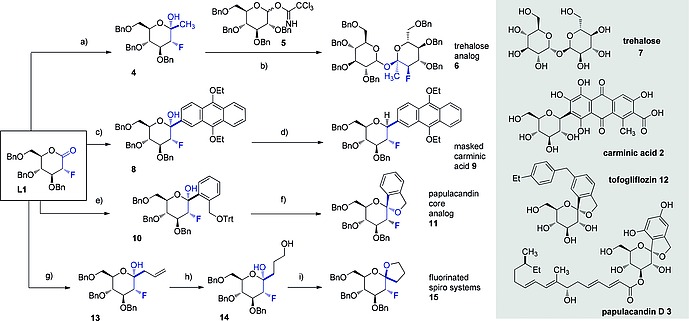
a) MeLi, THF, –78 °C, 30 min. quant. α/β = 1:0; b) 4, 5, TMSOTf, CH2Cl2, –78 °C to r.t. overnight, 30 %, αα/αβ = 1.8:1; c) nBuLi, ArBr (see SI), THF, –78 °C, 1 h, 73 %, α/β = 1:0; d) Et3SiH, BF3 ·Et2O, CH2Cl2/MeCN, –10 °C to r.t., 3 h, 80 %, α/β = 0:1; e) nBuLi, ArBr (see SI), THF/toluene (1:2), –78 °C, 1 h, 73 %, α/β = 1:0; f) Et3SiH, BF3 ·Et2O, MeCN, –40 °C to 0 °C, 2 h, 69 %, α/β = 1:0; g) Allylmagnesium bromide, THF, –78 °C, 30 min., 96 %, α/β = 1:0; h) 9‐BBN, H2O2 (30 % in H2O), NaOH (3 m aq.), overnight, r.t., 50 %; i) CSA, dioxane, 80 °C, 8 h, 53 %, α/β = 1:1.
