Scheme 1.
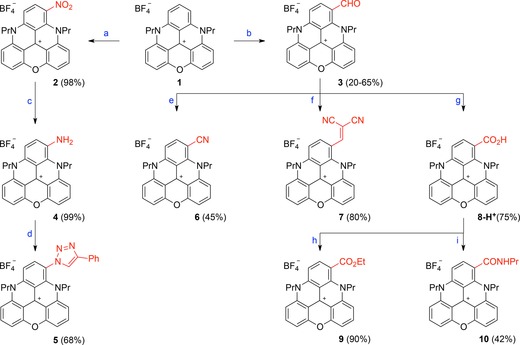
Synthesis of functionalized DAOTAs. Reagents and conditions: a) HNO3 (60 % aqueous solution), CH2Cl2, 25 °C, 12 h. b) POCl3 (24 equiv), DMF (12 equiv), 90 °C, 14 h. c) H2, Pd/C (20 mol %), CH2Cl2/MeOH, 25 °C, 30 min. d) tBuONO (1.5 equiv), trimethylsilyl azide (TMSN3; 2 equiv), CH3CN, 0 to 25 °C, 1 h, then PhC≡CH, CuSO4 ⋅5 H2O (0.1 equiv), ascorbic acid (0.2 equiv), NaHCO3 (0.2 equiv), CH3CN/H2O, 25 °C, 30 min. e) NaN3 (1.5 equiv), trifluoromethanesulfonic acid (TfOH; 3 equiv), CH3CN, 25 °C, 5 min. f) NCCH2CN (3 equiv), Ph3P (20 mol %), CH3CN, 130 °C (microwave (MW)), 25 °C, 1 h. g) NaH2PO4 (1 equiv), NaClO2 (2 equiv), H2O2, CH3CN, 60 °C, 1 h, then HBF4 (1 m aqueous solution). h) SOCl2 (6 equiv), CH2Cl2, 25 °C, 10 min, then EtOH (6 equiv), 25 °C, 10 min. i) SOCl2 (6 equiv), CH2Cl2, 25 °C, 10 min, then PrNH2 (18 equiv), 0 °C then 25 °C, 15 min.
