Figure 5.
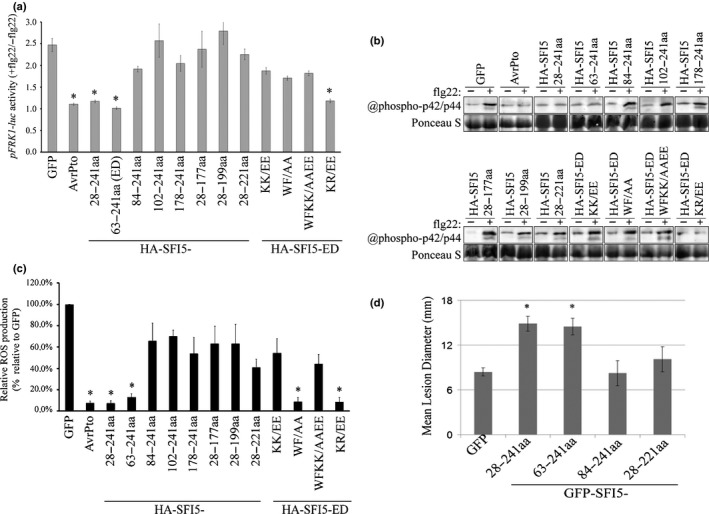
Both the calmodulin (CaM) binding motif and N‐terminal region of SFI5 are required for the suppression of flg22‐triggered early immune responses in tomato protoplasts and Phytophthora infestans infection in Nicotiana benthamiana. (a) Tomato protoplasts coexpressing HA‐tagged SFI5 deletion or point mutants with the two reporter genes pFRK1‐Luc and pUBQ10‐GUS were treated with or without flg22 (+/−flg22) and the pFRK1‐Luc activity was measured after 3 h. The promoter activity is calculated as the ratio of flg22‐induced luciferase activity relative to the untreated sample, which was normalized to the internal GUS activities (pFRK1‐Luc activity +flg22/−flg22). GFP and AvrPto served respectively as a negative and positive control for the suppression of pFRK1‐Luc activation by flg22. (b) Tomato protoplasts expressing HA‐tagged SFI5 deletion or point mutants were collected 20 min after flg22 treatment (+) or without flg22 treatment (−), and the phosphorylated mitogen‐activated protein kinases MAPK were detected by immunoblotting with the antibody raised against phosphorylated MAPK p44/p42. Ponceau S staining is shown as a loading control. (c) The oxidative burst in tomato protoplasts expressing HA‐tagged SFI5 deletion or point mutants is represented as the percentage of total photon counts measured between 6 and 20 min after flg22 treatment of the GFP control, which was set to 100%. GFP and AvrPto served respectively as negative and positive controls for the suppression of pFRK1‐Luc and MAPK activation and ROS burst by flg22. Data in (a, c) represent the mean ± SE from four independent experiments, for each of which three technical replicates were carried out. *, P < 0.05 (one‐way ANOVA followed by Dunnett's multiple comparison test). The results in (b) are representative of at least three independent experiments. (d) N‐terminal or C‐terminal deletion mutants of SFI5 were each transiently expressed via agro‐infiltration in one half of a N. benthamiana leaves and empty vector (GFP) was expressed in the other half. After 24 h, the infiltrated leaves were inoculated with P. infestans. Disease lesion diameters were measured 7 d post‐inoculation. Results are mean ± SE from three biological replicates, each of which used 24 leaves for inoculation per construct. Significant difference (*, P < 0.01) in lesion size compared to empty vector control was determined by one‐way ANOVA. GFP, green fluorescent protein.
