Figure 2.
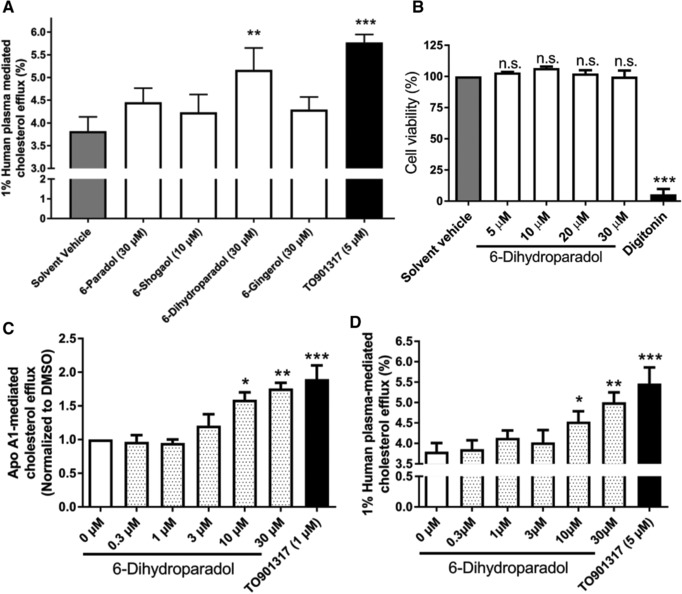
A) 6‐Dihydroparadol from ginger increases macrophage cholesterol efflux. THP‐1 cells were differentiated for 72 h with 200 nmol L−1 phorbol‐12‐myristate‐13‐acetate (PMA) and then loaded with cholesterol and radioactive cholesterol tracer ([3H]‐cholesterol) for 24 h. Cells were treated with 6‐paradol (30 μm), 6‐shogaol (10 μm), 6‐dihydroparadol (30 μm), 6‐gingerol (30 μm), TO901317 (5 μm, positive control), or the solvent vehicle (0.1% dimethyl sulfoxide [DMSO]) for another 24 h, then incubated with fresh serum‐free medium containing human plasma (1%, v/v) for 6 h, and cholesterol efflux was determined. B) 6‐Dihydroparadol does not affect cell viability of THP‐1‐derived macrophages. THP‐1 cells were differentiated as described in (A), and then loaded with unlabeled cholesterol for 24 h. Cells were treated with increasing concentrations of 6‐dihydroparadol (5–30 μm) for another 24 h. The viability was assessed by the resazurin reduction assay. Solvent vehicle treatment (0.1% DMSO) was used as a negative control. As a positive control, the cytotoxic natural product digitonin (50 μg mL−1, 4 h) was used. C,D) 6‐Dihydroparadol enhances both (C) apolipoprotein (apo) A1‐/ and (D) human plasma–mediated cholesterol efflux concentration dependently. THP‐1 cells were differentiated and loaded as described in (A). Cells were treated with increasing concentrations of 6‐dihydroparadol (0–30 μm) for 24 h. As a positive control, TO901317 (1 μm or 5 μm, 24 h) was used. Then macrophages were incubated with fresh serum‐free medium containing apo A1 (10 μg mL−1) or human plasma (1%, v/v) for 6 h, and cholesterol efflux was determined. The bar graphs represent mean ± SD from three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 versus control. n.s., not significant versus negative control (determined by Student's t‐test or ANOVA with Bonferroni's post hoc test).
