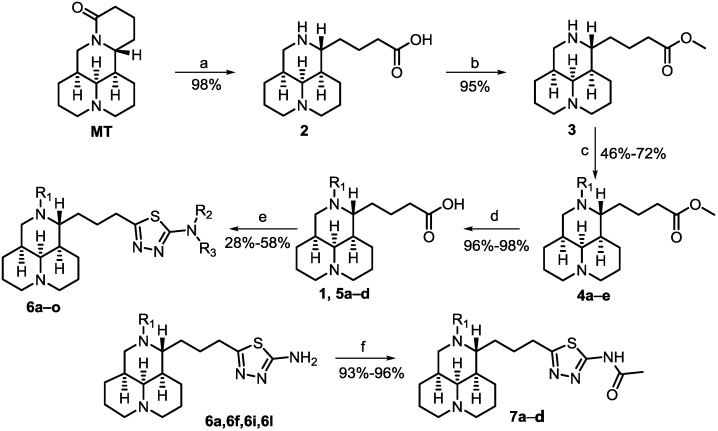
Scheme 1.
Reagents and conditions: (a) 5 N NaOH, reflux, 9 h; 10 N HCl, pH = 3–5; (b) 2 N MeOH/HCl, reflux, 2 h; (c) Substituted benzensulfonyl chloride or benzyl bromide, TEA or K2CO3, CH2Cl2 or MeCN, r.t., 6–8 h; (d) 5 N NaOH, reflux, 6 h; 10 N HCl, pH = 7–7.5; (e) P2O5/MsOH (1:5), thiosemicarbazide, 70 °C, 8 h; (f) Acetyl chloride, TEA, THF, r.t., 0.5 h.