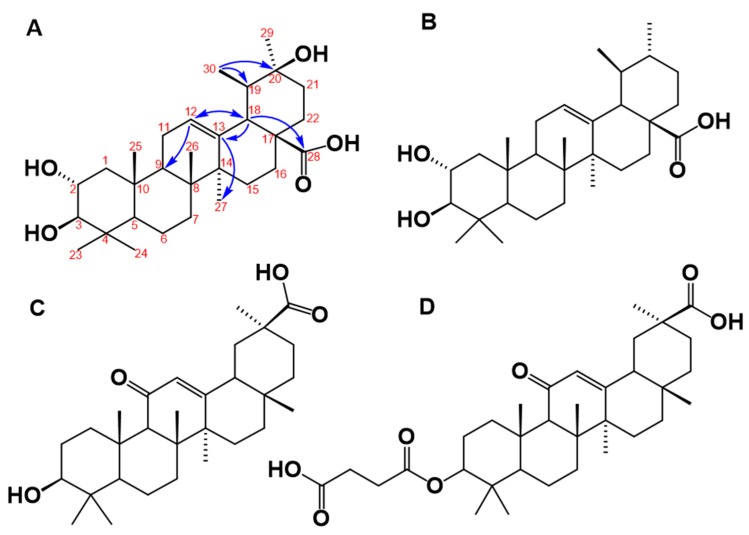
Figure 4.
(A) Proposed chemical structure of the triterpene molecule named isoyarumic acid isolated from C. telenitida. The numbers indicate carbon positions and the arrows are the most prominent correlations detected in the HMBC experiment. (B) Chemical structure of the related natural triterpene, corosolic acid, which is a reported inhibitor of 11β-HSD1. (C) Chemical structure of glycyrrhetinic acid. (D) Chemical structure of the synthetic molecule known as a carbenoxolone.