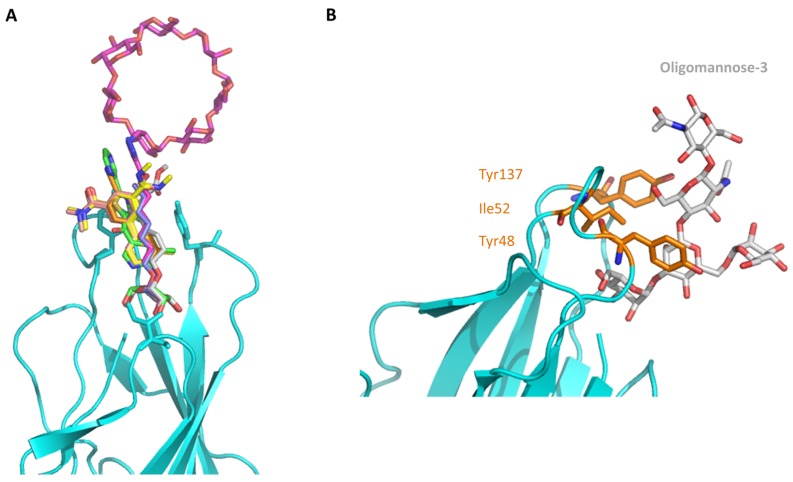
Figure 2.
FimH in complex with different inhibitors. (A) The mannose ring of different recent high-affinity inhibitors is bound similarly to FimH. Shown are the following inhibitors: HM (lilac, PDB ID: 4BUQ [31]), thiazolylaminomannoside (green; PDB code 5MTS [32], β-cyclodextrin-α-d-mannoside (purple; PDB code 5AB1 [23]), para-biphenyl-2-methyl-3′,5′di-methylamide-α-d-mannoside (yellow; PDB 5F2F [33]), 8-(Methoxycarbonyl)octyl-α-d-mannoside (grey, PDB code 4AVI [34]), 3′-Chloro-4′-(α-d-mannopyranosyloxy)biphenyl-4-carbonitrile (orange, PDB code 4CST [35]), para-biphenyl-2-methyl-3′-methylamidemannoside (rose, PDB code 5F3F [36]). (B) Oligomannose-3 bound to FimH. The mannoside is highlighted in grey and the tyrosine gate residues in yellow. The FimH lectin domain is shown in cyan in the cartoon (PDB code 2VCO [37]).