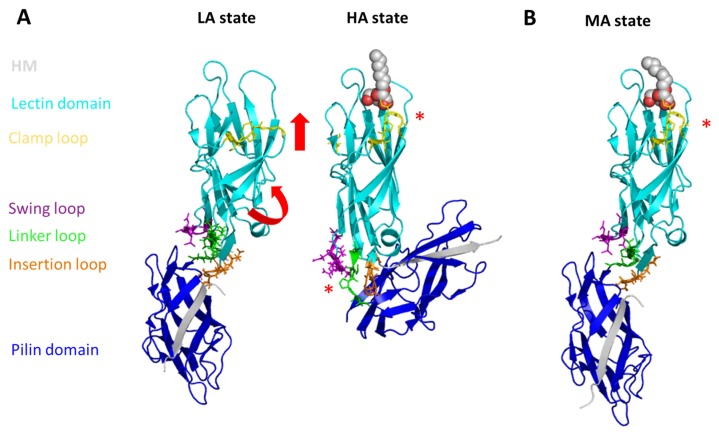
Figure 3.
The conformational flexibility of FimH. The LA (left; PDB code 4XOD), HA (middle; PDB code 4XOB), and MA (right; PDB code 4XOE, chain G and H) state are depicted. Following a β-sheet twisting mechanism, the lectin domain is elongated and straightened in the HA (MA) state (red arrows) leading to a local conformational change in the mannose-binding site (red star) locking it in its high affinity conformation. In the HA state the pilin domain is elongated and the link between the pilin and the lectin domain is weakened. The lectin (cyan) and the pilin (blue) domain as well as the clamp (yellow), swing (purple), insertion (orange), and linker (green) loops are shown in cartoon (domains, loops) and in lines (loops). The co-crystallized peptide is shown in grey cartoon. The HM bound to the HA and MA state is shown in van-der-Waals spheres (grey).