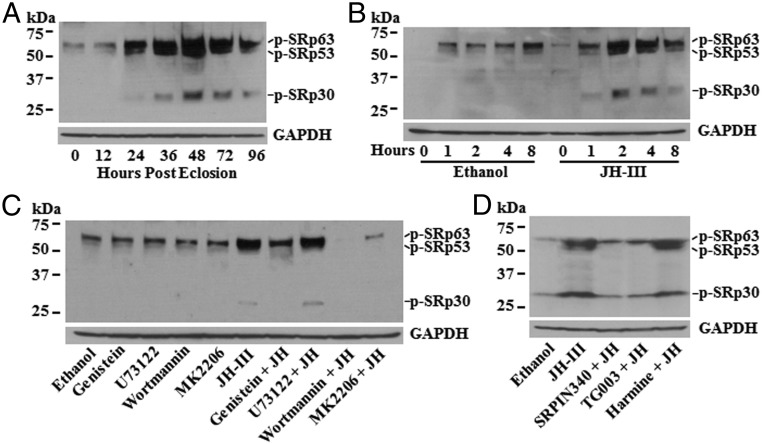
Fig. 1.
JH regulates the phosphorylation of mosquito SRSF proteins in the fat body after eclosion. (A) Phosphorylation of SRSFs in female adults at 0, 12, 24, 36, 48, and 72 h PE was detected by immunoblotting. Equal amounts of proteins were loaded on SDS/PAGE and were blotted with an antibody (Clone 1H4; EMD Millipore) that binds to a phosphoepitope in SR proteins (p-SR). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. (B) Phosphorylation of SRSFs was regulated by JH in the fat body of newly emerged mosquitoes. Fat bodies were isolated from female mosquitoes at 30 min PE and were cultured in vitro in the presence of JH-III (1 μM) for 0, 1, 2, 4, and 8 h. The phosphorylation of SRSFs was monitored by immunoblotting. (C) RTK/PI3K/Akt pathway was required for the JH-regulated SRSF phosphorylation. In vitro-cultured fat bodies were preincubated with genistein, U73122, wortmannin, and MK2206 (the specific inhibitors of RTK, PLC, PI3K, and Akt, respectively) for 1 h, followed by incubation with JH-III (1 μM) for 4 h. Equal amounts of proteins from each treatment were used for immunoblotting analysis. (D) SRPK and Clk were primarily responsible for the JH-regulated phosphorylation of SRSFs. SRPIN340, TG003, and harmine were used in the pretreatment to inactivate SRPK, Clk, and DYRK, respectively. The phosphorylation of SRSFs in the cultured fat bodies was assessed by immunoblotting after incubation with JH-III.