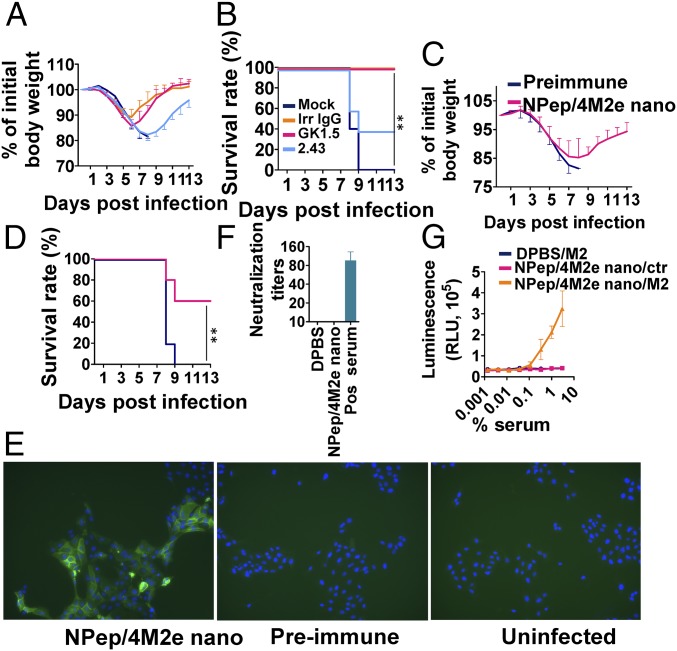
Fig. 5.
Protective mechanisms. (A) Morbidity and (B) mortality of clone GK1.5-treated CD4+-depleted or clone 2.43-treated CD8+-depleted BALB/c mice that were IM immunized twice were evaluated after lethal dose 6 × mLD50 infection with H5N1. Antiflagellin IgG1 clone X5A12 was used as an irrelevant negative control, and DPBS was used for mock-treatment. (n = 5) (C) Morbidity and (D) mortality of serum passively transferred BALB/c mice were evaluated after lethal dose 6 × mLD50 infection with H5N1 (n = 5). (E) Immunofluorescence staining of H5N1-infected MDCK cells using preimmune and NP-peptide/4M2e–layered nanoparticles (NPep/4M2e nano) immune serum as the primary antibodies and then Alexa Fluor 488 Goat Anti-Mouse IgG as the secondary antibody. The uninfected MDCK cells incubated with NP-peptide/4M2e–layered nanoparticle immune serum were the negative control. (Magnification, 10× objective.) (F) Viral neutralization potency of immune sera was evaluated in a standard neutralization assay against H5N1. Convalescent mouse serum from H5N1-infected mice was used as a positive control. (G) ADCC surrogate assay with pooled prechallenge sera from NPep/4M2e–layered nanoparticle and DPBS immunization groups and HEK293T cells stably expressing M2. The nontransfected HEK293T cells were used as the negative control (n = 3). Data are presented as mean ± SD. Statistical significance was analyzed by t test for F and G. P values shown in bar charts and N.S. indicates no significance between two compared groups. The statistical analysis of survival rate difference between the antibody-treatment group and the mock-treatment group in B and D was performed with a log-rank test (**P < 0.01; n = 5). The experiments of (E) immunofluorescence staining and (F) viral neutralization assay were repeated twice with similar results.