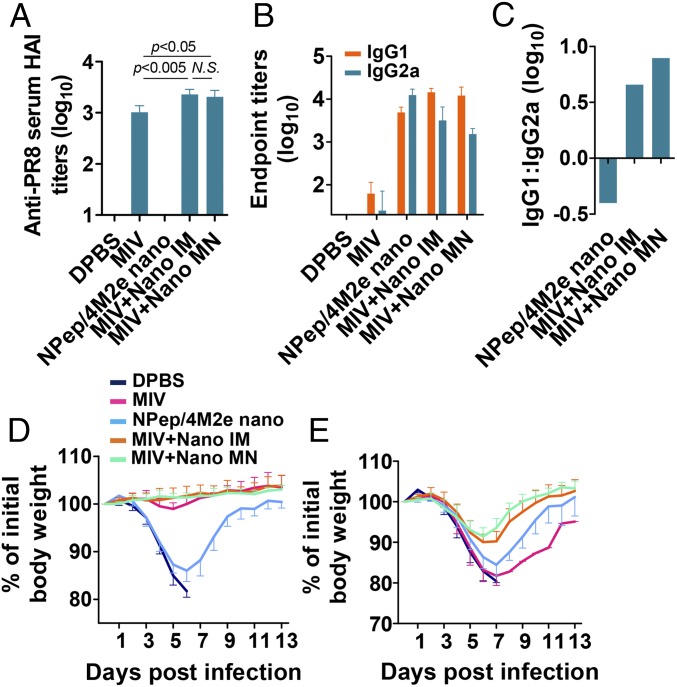
Fig. 7.
Immunity induced by supplementation of monovalent inactivated PR8 vaccine (MIV). IM immunization groups include 10 μg NP-peptide/4M2e–layered nanoparticles (NPep/4M2e nano), 10 μg MIV, 10 μg MIV plus 7.5 μg NP-peptide/4M2e–layered nanoparticles (MIV+Nano IM), 10 μg MIV plus MN patch containing 7.5 μg NP-peptide/4M2e–layered nanoparticle (MIV+Nano MN), or 50 μL DPBS as a placebo. (A) Anti-PR8 serum HA inhibition titers of serum collected 3-wk postboosting immunization (n = 5). (B) Determination of IgG1 and IgG2a titers against M2e using ELISA method (n = 8). (C) Bar chart showing logarithm values of the ratio IgG1: IgG2a. Morbidity of immunized BALB/c after lethal dose 6 × mLD50 infection with PR8 (D) and Phi (E). Data are presented as mean ± SD in A, B, D, and E. Statistical significance was analyzed by t test for A. P values shown in bar charts and N.S. indicates no significance between two compared groups.