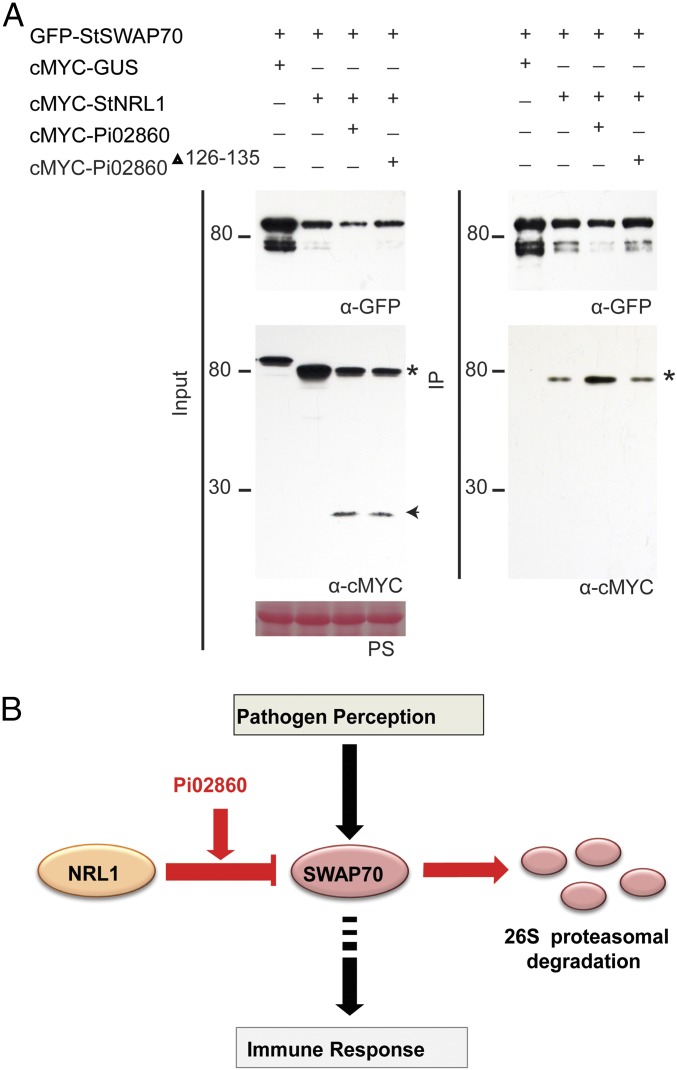
Fig. 6.
Pi02860 enhances StNRL1 and StSWAP70 association in planta. (A) IP of protein extracts from N. benthamiana leaves transiently expressing GFP-StSWAP70 with either cMYC-GUS, cMYC-StNRL1, cMYC-StNRL1 + cMYC-Pi02860, or cMYC-StNRL1 + cMYC-Pi02860Δ126–135 using GFP-Trap shows reduced protein levels of GFP-StSWAP70 in the presence of cMYC-StNRL1, which are further decreased when coexpressed with both cMYC-StNRL1 and cMYC-Pi02860 but not with cMYC-StNRL1 + cMYC-Pi02860Δ126–135. Increased cMYC-StNRL1 protein pull downs with GFP-StSWAP70 in the presence of cMYC-Pi02860 indicates that Pi02860 enhances the association between StSWAP70 and StNRL1. Expression of constructs in the leaves is indicated by a “+.” Protein size markers are indicated in kilodaltons, and protein loading is indicated by Ponceau stain. Immunoblots with anti-GFP shows protein fusion of GFP-StSWAP70 and the anti-cMYC shows protein fusion of cMYC-StNRL1 (star) and cMYC-Pi02860 (arrow) of the expected size. (B) Model for the action of Pi02860 and NRL1 (red) on immunity regulated by SWAP70 (black). Perception of pathogen molecules (such as the PAMP INF1) leads to the activation of SWAP70 which, in turn, positively regulates immunity (24, 25). NRL1, an S factor and predicted CRL ubiquitin E3 ligase component (22), negatively regulates immunity by mediating proteasome-dependent degradation of SWAP70. Effector Pi02860 enhances interaction between NRL1 and SWAP70, promoting increased turnover of SWAP70 to suppress immunity.