Significance
Only two infectious diseases, smallpox in humans and rinderpest in cattle, have been eradicated so far. Peste de petits ruminants (PPR), a viral disease representing a major burden for sheep and goat farmers across Africa and Asia, is now targeted for eradication through mass vaccination campaigns. While an efficacious vaccine providing protective and lifelong immunity exists, the level of PPR virus transmission in animal populations is unknown. By combining the results from a nationwide serological survey with a dynamic model simulating viral spread, we estimated viral transmission potential in Ethiopia, where PPR virus is endemic, and vaccination coverage required for disease elimination. This approach is relevant to identify populations at high risk of viral persistence and to inform vaccination strategies.
Keywords: eradication, elimination, mathematical model, vaccination, control
Abstract
Peste des petits ruminants (PPR), a devastating viral disease of sheep and goats, has been targeted by the global community for eradication within the next 15 years. Although an efficacious attenuated live vaccine is available, the lack of knowledge about the transmission potential of PPR virus (PPRV) may compromise eradication efforts. By fitting a metapopulation model simulating PPRV spread to the results of a nationwide serological survey in Ethiopia, we estimated the level of viral transmission in an endemic setting and the vaccination coverage required for elimination. Results suggest that the pastoral production system as a whole acts as a viral reservoir, from which PPRV spills over into the sedentary production system, where viral persistence is uncertain. Estimated levels of PPRV transmission indicate that viral spread could be prevented if the proportion of immune small ruminants is kept permanently above 37% in at least 71% of pastoral village populations. However, due to the high turnover of these populations, maintaining the fraction of immune animals above this threshold would require high vaccine coverage within villages, and vaccination campaigns to be conducted annually. Adapting vaccination strategies to the specific characteristics of the local epidemiological context and small ruminant population dynamics would result in optimized allocation of limited resources and increase the likelihood of PPR eradication.
Peste des petits ruminants (PPR) is a disease of sheep and goats caused by a morbillivirus closely related to rinderpest virus. Highly transmissible, the disease has a devastating impact on small ruminants, as morbidity and mortality rates can reach near 100% in naive populations (1, 2). PPR virus (PPRV) is now endemic in most of Africa and throughout Asia, where it is one of the main constraints to small ruminant production and welfare, and therefore a threat to food security and livelihoods of the poorest communities, for which sheep and goats are often an important asset. Moreover, PPRV spillover from domestic to wild populations resulted in serious concerns for the conservation of some critically endangered species (3–6).
In the aftermath of the eradication of rinderpest, the World Organization for Animal Health and the Food and Agriculture Organization of the United Nations launched an initiative to eradicate PPR by 2030. The global strategy (7) heavily relies on the immunization of small ruminant populations through the organization of mass vaccination campaigns, due to the availability of an efficacious attenuated live vaccine producing lifelong immunity against all PPRV serotypes after a single administration (8). Such campaigns are, however, costly and difficult to implement in the field due to the vaccine’s thermolability (8), the accessibility and mobility of some small ruminant populations, and the lack of precise census data and national animal identification systems. To reduce the costs of eradication efforts, it is essential to assess the PPRV transmission potential, so small ruminant populations acting as a viral reservoir can be targeted, and within them, the minimal fraction of animals that needs to be immunized to prevent viral transmission can be estimated (7). Such information is, however, missing.
Among PPRV-endemic countries, Ethiopia has the seventh largest small ruminant population (FAOSTAT; www.fao.org/faostat/en/#home), which accounts for a substantial fraction of national demand for meat consumption and export earnings (9–11). PPR was first clinically suspected in the country in 1977, before serological and virological evidence of its presence were documented in 1984 and 1991 (12, 13). Before the first mass vaccination campaign, a nationwide serological survey was initiated in 1999 (13). By fitting a metapopulation model of PPRV transmission to these survey results, this study aims to estimate the level of PPRV transmission within and between Ethiopian small ruminant village populations, and the optimal vaccination coverage required for disease elimination.
Results
Estimation of Transmission Parameters.
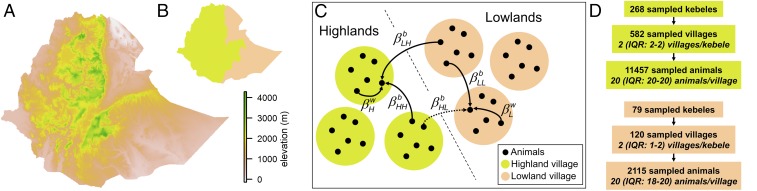
The model simulated the spread of PPRV between small ruminants—sheep and goats—within and between Ethiopian villages. The relatively small number of small ruminants in an average village meant that PPRV did not persist at village level, but did at the metapopulation level, through a “rescue effect” (14). Variation in the Ethiopian landscape defines different agroecological zones associated with distinct livestock husbandry systems. While sedentary mixed livestock–crop farms prevail in the high central plateau, regions of low elevation are home to pastoralists, who heavily rely on livestock production for their livelihood (Fig. 1 A and B) (15). Small ruminant flocks are notably larger and more mobile—in search for grazing and watering points—in the lowland pastoral than in the highland sedentary systems (10, 16–18). Modeled village populations were thus classified as sedentary or pastoral, and associated with different transmission potential. As illustrated in Fig. 1C, the within-village transmission parameters and referred to the number of effective contacts per unit of time (i.e., contacts that would result in infection if involving a susceptible and an infected small ruminant) made by a small ruminant with other small ruminants in the same village in highlands and lowlands, respectively. PPRV also spread between villages through population mixing at watering points or pasture, and through live-animal trade. was the number of effective contacts per unit of time that a small ruminant in a village in region r made with small ruminants from other villages of region k. Therefore, , , and referred to PPRV transmission between lowland villages, between highland villages, and from lowlands to highlands, respectively (Fig. 1C). accounted for transmission from highlands to lowlands and was expressed as , with , the relative strength of mixing (if contacts were reciprocal, ), and , the ratio between highland and lowland population sizes. While intervillage contacts resulting from mixing at watering points and pastures were reciprocal, this was not the case with live-animal trading. It was strongly directed from lowlands, where prices and the humans-to-small ruminants ratio are low, into highlands, where prices and the humans-to-small ruminants ratio are high (10, 19, 20), suggesting that . When estimated along other transmission parameters, was poorly determined, as its marginal posterior distribution remained similar to its prior. We fixed , assuming that PPRV transmission from highlands to lowlands was epidemiologically negligible. This scenario maximized intervillage transmission in lowlands as and were negatively correlated. An alternative scenario, with , is presented in SI Appendix. We used an approximate Bayesian computation method based on a sequential Monte-Carlo algorithm (ABC-SMC) to sample from the joint posterior distribution of the transmission parameters (21–24). This likelihood-free approach relies on matching a set of summary statistics (Methods) obtained from model simulations to the results of the serological survey (13). As such, the output of our ABC-SMC inference is actually an approximation of the posterior distribution, but for convenience it will be referred to as the posterior distribution throughout the text. The survey covered 7 of the 11 regions (first administrative division) and 84 of the 546 weredas (third administrative division) into which Ethiopia was divided. Out of 11,457 and 2115 samples collected in highlands and lowlands (Fig. 1D), 4.6% and 16.6% were positive. As the village of origin was not specified for most samples (SI Appendix), the proportion of positive animals within a kebele (subdistrict, fourth administrative division) was reported (Fig. 2F).
Fig. 1.
Model structure and serological survey coverage. (A) Elevation in Ethiopia. (B) Division into lowlands (Afar and Somali regions) (brown) and highlands (green). (C) Modeled animals are grouped into villages, which are differentiated as lowlands or highlands. refers to PPRV transmission within a village in region r, and to intervillage transmission from region k to r. An arrow is dashed as was set to 0. (D) The number of sampled units.
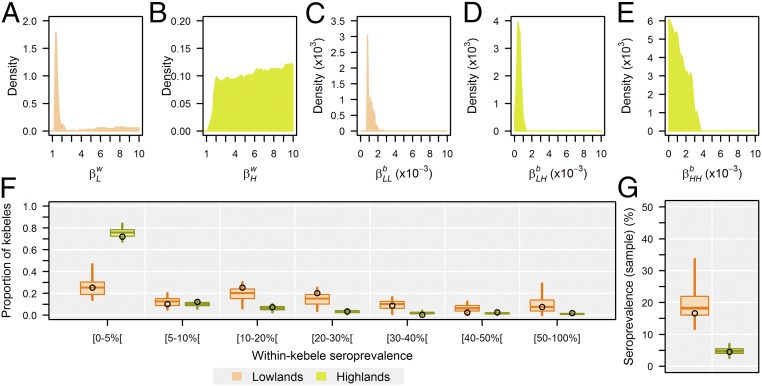
Fig. 2.
Marginal posterior distributions and comparison between simulated and observed survey results. (A–E) Marginal posterior distributions: the number of effective contacts/animal within (A) a lowland village and (B) a highland village; the number of effective contacts/animal (C) between lowland villages, (D) from the lowlands into the highlands, and (E) between highland villages. (F) Observed (dots) and posterior predictive (boxplots) distributions of surveyed kebeles according to their seroprevalence in the lowlands (brown) and highlands (green). (G) Observed (dots) and posterior predictive (boxplots) proportions of seropositive surveyed small ruminants; boxplots show the 5th, 25th, 50th, 75th, and 95th percentiles.
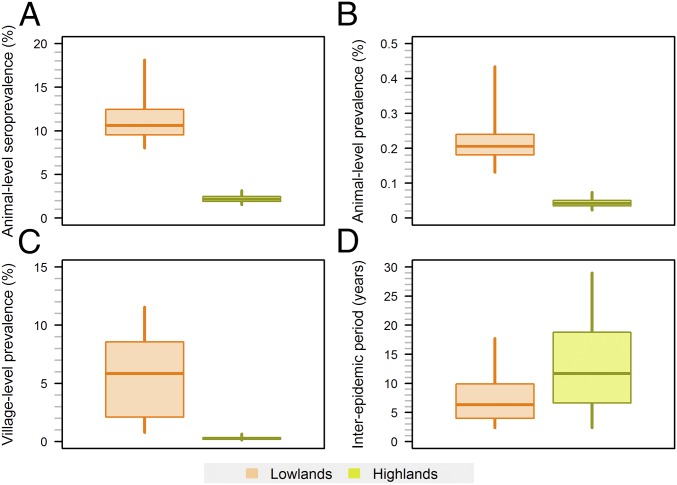
Model simulations adequately reproduced the serological survey results in both areas (Fig. 2 F and G). Nevertheless, the proportion of surveyed kebeles with low seroprevalence (<5%) in highlands and with a seroprevalence ranging between 11 and 30% in lowlands were respectively overestimated and underestimated. By the time the serological survey was implemented, 20–25 y following the first PPRV incursion, the simulated animal-level seroprevalence already fluctuated around its long-term average (SI Appendix, Fig. S1). The animal-level prevalence of infection was on average five times higher in lowlands than in highlands. Likewise, epidemics were more frequent in lowland than in highland villages (Fig. 3).
Fig. 3.
Posterior predictive distribution of (A) animal-level seroprevalence, (B) animal-level viral prevalence, (C) village-level viral prevalence, and (D) interepidemic periods. Young and adults were considered. A village was classified as infected if the prevalence ≥0.5%. Interepidemic period: the length of time between two successive epidemic peaks in a given village. The 5th, 25th, 50th, 75th and 95th percentiles are shown.
Marginal posterior distributions of transmission parameters are presented in Fig. 2 A–E, and summarized in Table 1. SI Appendix, Fig. S6 shows the posterior predictive distributions of village-level reproduction numbers. The highest posterior density of the level of within-village transmission in lowland was concentrated at low values (range, 1.2–2.4) with the maximum a posteriori equal to 1.37 (Fig. 2A). However, a second, low-probability, and almost-uniform mode was located at high values. Indeed, for > 5, the model simulations were insensitive to further increases in (SI Appendix, Fig. S2), until it reaches the upper bound of the prior distribution. The identifiability of highland transmission parameters was limited, with a trade-off between intervillage transmission routes from other highland or lowland villages, and being negatively correlated (SI Appendix, Fig. S5). While this lack of identifiability prevented us from precisely inferring actual highland parameter values, the joint posterior distribution was restricted to a region of the parameter space corresponding to the highland village-level reproduction number , suggesting that PPRV could not be maintained within highlands, but only within lowlands (i.e., ) (Table 1). PPRV incursions into highlands would ultimately fade out unless the virus was reintroduced.
Table 1.
Parameter prior distributions and posterior estimates, and posterior predictive values of reproduction numbers
| Parameter | Prior | Posterior median (95% CrI) |
| U[1,10] | 1.56 (1.26–9.45) | |
| U[1,10] | 6.19 (1.85–9.73) | |
| (×10−3) | U[0,10] | 1.08 (0.75–1.94) |
| (×10−3) | U[0,10] | 0.50 (0.08–1.05) |
| (×10−3) | U[0,10] | 1.19 (0.06–3.47) |
| — | 1.54 (1.24–9.35) | |
| — | 6.11 (1.83–9.63) | |
| — | 1.49 (1.27–2.01) | |
| — | 1.09 (0.20–2.36) | |
| — | 0.32 (0.01–0.91) |
β is the number of effective contacts per animal over a 10 d-period—the length of the infection period; refers to PPRV transmission within a village in region r, and to intervillage transmission from region k to r; likewise, is the within-village reproduction number in region r, and the village-level reproduction number from region k to r; and U is uniform distribution. Median and 95% credible interval (Crl) were computed.
Immunity Threshold and Vaccination Coverage.
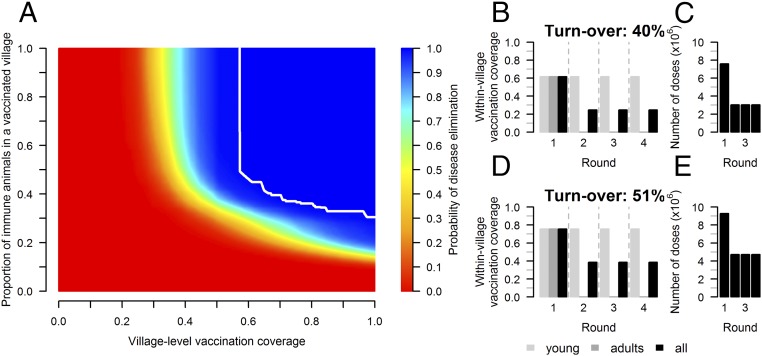
This source–sink dynamics suggests that vaccinating lowland populations could eliminate PPRV in both regions. The lowest fraction of immune animals preventing PPRV spread for all values of the posterior distribution was reached if pa = 37% of small ruminants in pv = 70.7% of villages were protected against infection (Fig. 4A). When ignoring immunity resulting from past infection, keeping the immunity level above pa within a village for a whole year would require the immunization of 61.7% of animals, adults and young, as 40% of the population was renewed every year under the baseline scenario (Fig. 4B). Over subsequent years, annual campaigns immunizing 61.7% of young animals born since the preceding campaign would prevent PPRV spread. Vaccination programs are recommended to be run over a 3- to 4-y period (7). Maintaining the immunity level above the elimination threshold for a period of 4 y would suppress PPRV circulation, and requires 16.6 million animals to be vaccinated in lowlands, assuming that the vaccine results in complete and lifelong immune protection in all vaccinated animals (Fig. 4C). Compared with a vaccination strategy targeting all lowland small ruminants during the first round and all young small ruminant during subsequent rounds, the number of required vaccine doses would be reduced by 56.4%. However, as turnover increased (Fig. 4 D and E) and vaccine effectiveness decreased (SI Appendix, Table S5), the required vaccination coverage rose. Assuming that only 80% of vaccinated animals are effectively immunized meant a 25% increase in coverage. Variation in the number of villages, PPR case fatality rate, and demographic parameters did not have a major impact on infection parameter estimates and immunization thresholds required for PPRV elimination (SI Appendix).
Fig. 4.
Immunity threshold and vaccination coverage to eliminate PPRV in lowlands. Vaccine-induced immunity was assumed to be fully protective against infection. (A) Probability of PPRV elimination as a function of the proportion of vaccinated villages and the proportion of immunized animals in each vaccinated village; the white solid line corresponds to a probability of PPRV elimination of 1. (B) Proportion of a village population to be vaccinated during each yearly campaign to maintain the immunity level above the elimination threshold (37%) under the baseline scenario—40% of the population was renewed each year. (D) Same with a turnover of 51%. (C and E) Number of vaccinated animals/year.
Discussion
Model outputs suggest that PPRV transmission was sustained in Ethiopia’s lowland pastoral region through viral transmission between small ruminant village populations. Lowlands thus acted as a reservoir of infection from which PPRV spilled over into the highland sedentary region where its maintenance was unlikely. The trade of sheep and goats from Ethiopian lowlands into neighboring countries and Gulf states (17, 19, 25) makes PPRV elimination from Ethiopia’s lowlands not only a national, but also a regional and even global priority. Based on our estimation, ensuring that at least 37% animals were immune in at least 70.7% of pastoral villages would prevent PPRV spread. However, due to the high population turnover and not all vaccinated animals developing a protective immunity, vaccination coverage would need to be substantially higher (26). Potential causes for inadequate immunity may include individual variations in immune response, improper vaccine administration, and the use of ineffective vaccine batches. Currently available vaccines are thermolabile (8), requiring the maintenance of the cold chain until their administration. This is a major challenge as most PPR-endemic countries have poor infrastructure and periods of hot climatic conditions. The recent development of a thermostable presentation would facilitate vaccine delivery (27). The model assumed random selection of vaccinated villages and animals. If selection was purposive, for example, based on accessibility, higher coverage would be needed to prevent persistence of the infection in unvaccinated population clusters. The number of animals to be vaccinated each year seems achievable: apart from the first vaccination round, it was lower than the coverage achieved during each annual mass vaccination campaign conducted between 2005 and 2011 in the whole country (11). Although an economic analysis would be required to assess the most cost-effective strategy balancing the overall number of vaccine doses used and the number of vaccinated villages, vaccination efforts would be lower than suggested by the global control and eradication strategy (7), which recommended the vaccination of almost all small ruminants above 3 mo of age. The authors of the strategy, however, recommended adapting this generic strategy to local conditions, and emphasized that targeting at-risk populations, especially pastoral flocks, and estimating context-specific elimination thresholds would reduce eradication costs.
The model suggested frequent PPRV incursions into highlands from lowlands. In the search for grazing and watering points, pastoral flocks may move toward highlands, where they then mix with sedentary flocks. Moreover, goats and sheep traded from lowlands into highlands are moved through several markets, over long distances (10). Such marketing systems are likely to promote viral amplification, as observed with other species (28). While most animals traded from lowlands would end up in abattoirs, they could infect highland animals brought to markets. Unsold highland animals returning to their village of origin could then spread the infection. If these interfaces between pastoral and sedentary populations were characterized spatiotemporally, they could be targeted by vaccination to reduce viral spillover. The level of PPRV transmission from highlands to lowlands was uncertain, but likely to be low (SI Appendix). By fixing , we prioritized the worst-case scenario, maximizing PPRV transmission potential within lowlands, and therefore the elimination threshold.
The inference about PPRV not being sustained in highlands is consistent with the national strategy (11): mass vaccination campaign in lowlands and ring vaccination following PPR outbreaks in highlands. Although the model assumed that highland villages were homogeneous, it is likely that in reality population structures and husbandry practices are heterogeneous across this large area, where most of the Ethiopian human population lives. Such heterogeneity could result in spatial variation in PPRV transmission potential, creating population pockets acting as viral reservoirs. As specified in the national plan (11), the vaccination strategy should be revised as new evidence becomes available. Although Ethiopia is mainly an exporter of small ruminants (17, 19, 25), cross-border movements of pastoralists can occur, triggered by water and pasture scarcity (17). In this context, any success with PPRV elimination in Ethiopia may be temporary, as it is likely to be followed by reincursion from infection reservoirs across the border. Vaccination programs therefore need to be coordinated regionally, across countries connected via PPRV transboundary transmission routes.
As mentioned above, for > 5, the model simulations were insensitive to further increases until it reaches the upper bound of the prior distribution. Caution should thus be applied when interpreting median and credible interval, which would increase with wider prior distributions (SI Appendix). We emphasized the lack of model identifiability for highland transmission parameters. Nevertheless, although the data were not informative enough to obtain tight posterior distributions for these parameters, they consistently excluded regions of the parameter space corresponding to . Therefore, from an elimination perspective, higher precision of those parameter estimations was unnecessary. Although additional data could help refining our parameter estimates, the present results already allow us to considerably narrow down the range of suitable options among all possible vaccination strategies. If new large-scale serological surveys were conducted, timing of successive vaccination campaigns would need to be accounted for, as it is not possible to discriminate infection- and vaccine-induced immune responses (8). The age of sampled animals should also be recorded systematically and consistently, as these data would be very useful for refining parameter estimates.
The validity and relevance of this study relied on several assumptions. One of them was that PPRV had reached an endemic state at the time of the serological survey. This assumption was consistent with model simulations and supported by genetic evidence suggesting that viral lineages circulated decades before their detection (29). It was also assumed that (i) the serological survey was representative of the epidemiological situation in lowlands and highlands, and (ii) current and 1999 PPRV transmission potential were similar. Probabilistic sampling is challenging in countries with limited infrastructure. Selection bias due to nonrandom selection of some surveyed populations and animals might have occurred (13), influencing the observed seroprevalence patterns, and therefore transmission parameter estimates. Other serological surveys conducted in Ethiopian pastoral flocks reported a seroprevalence of 12% in 2001 (30), similar to the 1999 survey results, and a seroprevalence of 31% in 2009–2010 (31). This increase—which corresponded to the upper limit of the simulated seroprevalence—may result from the limited number of villages and geographical area covered by that survey, or the incorrect reporting of the vaccination status of sampled animals by farmers. It may also reflect actual changes in PPRV epidemiology. Until PPRV lineage IV was detected in Ethiopia in 2010, only lineage III was thought to circulate in the country (32). Although the timing of lineage IV introduction and the relative prevalences of lineage III and IV are uncertain, the suspected higher virulence of lineage IV (33) may be associated with a greater transmission potential, meaning that the elimination threshold might have been underestimated. Moreover, the successive vaccination campaigns might have impacted on the evolution, and transmission potential, of local PPRV strains.
Given the diversity of PPRV strains, small ruminant breeds, population densities, and trading and farming practices across Asia and Africa, caution needs to be exercised when attempting to generalize these results. Indeed, variation in seroprevalence patterns across different geographical and epidemiological settings (34–38) may be caused by varying levels of PPRV transmission. Susceptibility has been reported to vary by species, with goats being generally considered to be more susceptible than sheep (1, 35, 37, 38), and even by breeds (39). However, similar or higher levels of susceptibility in sheep than goats are also documented (34, 36). It is therefore important to quantify potential variation in infectiousness, as it would affect optimal vaccination coverage. Although other domestic (1, 2, 35) and wild (5, 40) animal species are susceptible to PPRV, current knowledge suggests that control of the infection in small ruminants would prevent disease outbreaks in other species (6, 41, 42), as observed with rinderpest following its control in cattle.
Another limitation of the model was the lack of reliable small ruminant population data, especially for pastoral flocks, and the lack of specific data about spatiotemporal variation in population sizes and demographic profiles, farming and trading practices. Although pastoralists prevail in Afar and Somali regions, and sedentary flocks in the other parts of the country, production systems are more diversified and their distribution more heterogeneous than assumed in the model (10, 17). Lowland pastoral populations outside Afar and Somali were only subject to limited sampling in the 1999 survey, but they should be included in vaccination programs targeting pastoral flocks. As live-animal trade networks are consistently highly heterogeneous in multiple settings (43, 44), this is also likely to be the case for Ethiopian small ruminants (45). Identifying and targeting the most at-risk populations at a higher spatial resolution would allow further reducing the required vaccination coverage (46), but this needs detailed data on demographic processes, including their spatiotemporal variation (47). Moreover, the way in which village populations are repopulated in the aftermath of an outbreak is not documented, but it is of importance to understand the speed at which susceptible populations are replenished as this may promote PPRV endemicity. Seasonality in infection patterns and population dynamics were not explored. However, seasonal variation in environmental conditions affects the availability of grazing, and consequently demographic processes (e.g., variations in birth rates during the year in some husbandry systems), animal movements, mixing patterns within and between husbandry systems (17, 18), and therefore PPRV transmission. Likewise, trade patterns are likely to vary according to seasonal religious or other festivals, as observed for other livestock species and countries (48, 49). Accounting for these seasonal patterns would allow the identification of the most suitable time periods for vaccination.
In conclusion, identifying and targeting high-risk populations through vaccination campaigns informed by the estimation of context-specific PPRV transmission levels would not only reduce the cost of PPR eradication, but by setting more achievable vaccination coverage also increase the likelihood of success. Further information would be needed on spatiotemporal variation in PPRV distribution and small ruminant population dynamics to more precisely identify high-risk populations, to refine optimal vaccination coverage and to identify the most suitable time periods during which to vaccinate.
Methods
Small Ruminant Population Data.
As an estimated 80–90% of pastoralists were grouped in the two eastern regions of Afar and Somali (50, 51), sheep and goats in Afar and Somali regions are here referred to as the pastoralist, lowland, small ruminant population, and sheep and goats in the rest of the country as the sedentary, highland, small ruminant population (Fig. 1 A and B). Partitioning sedentary and pastoral systems according to an elevation threshold of 1,000 m (10) did not affect seroprevalence patterns (SI Appendix, Table S1). In the serological survey to which the model was fitted, the sampling of lowland populations in the south and west of the country was very limited. Serological results for regions other than Afar and Somali thus reflected infection patterns in their highland areas. To our knowledge, a census of Ethiopian villages was not available. We estimated plausible values based on the literature and the human population census. In the main text, we considered 10,000 lowland and 100,000 highland villages. Alternative scenarios are detailed in SI Appendix. The number of small ruminants in highlands and lowlands was estimated at 27.2 and 17.4 million, respectively (52, 53) (SI Appendix).
Model.
PPRV transmission within a village.
While goats are sometimes reported to be more susceptible to PPRV than sheep (1), the results of the serological survey did not suggest any difference in the seroprevalence between both species (13). We did not, therefore, differentiate sheep and goats, using small ruminant as the unit of the model. All villages were similar according to their region of origin. Highland and lowland villages only differed in their population size and PPRV transmission potential. As production was extensive and animals from multiple flocks could mix within a village, homogeneous mixing was assumed within villages. PPRV dynamics within a village was first explored using a stochastic model (SI Appendix). For the investigated range of small ruminant population sizes, a PPRV incursion caused an epidemic followed by extinction, that is, it could not become endemic. To reduce computing time, within-village PPRV transmission was modeled as a deterministic process. Viral fade-out was simulated by setting the number of infected animals to 0 when the epidemic curve reached its trough following the epidemic peak. The number of infectious animals then remaining in the village depended on the population size and the level of PPRV transmission, but it was always lower than 3.5, in agreement with the high risk of fade-out observed in the stochastic simulations.
Small ruminants were divided into two age categories, young (<1 y old) and adults (>1 y old), which differed in their non–PPR-related mortality rates, and . New sheep and goats entered into the village i, through births, which occurred all year long, as breeding was generally uncontrolled (10, 54). Small ruminants could pass through three successive and mutually exclusive health states: susceptible, infected, and recovered. Susceptible animals became infected following an effective contact with an infected small ruminant. Infected small ruminants could either survive and acquire lifelong immunity to PPR (1), or die due to the disease. We did not discriminate the infection state into latency (i.e., infected but not infectious) and infectiousness as the model was run in discrete time, with the duration of a time step being equal to the length of the infection period (i.e., assuming a fixed infection period for all animals). The number of susceptible (), infected (), and recovered () small ruminants of age a in a village i in a region r (highlands or lowlands) at time t were expressed by the difference equations below. The rates of demographic processes being much lower than the transmission rate, they were approximated as follows:
The subscripts a = 1 and a = 2 referred to the first (young) and second (adult) age categories. referred to viral incursion (see below). referred to the rate at which young small ruminant became adults. was the PPR case fatality rate. In the absence of disease, all villages from a given region r were composed by small ruminants, and their birth rate was constant . As PPR caused abortion and mortality (1, 2), the birth rate was reduced during an epidemic: , with being the proportion of adult small ruminants. Once the epidemic faded out in village i, , ensuring the progressive replenishment of the village population. Finally, was the risk of infection for a susceptible small ruminant due to contacts with infected small ruminants in the same village i: . Production being extensive, the contact process was assumed to be frequency-dependent, with being the total number of small ruminants in village i at time t. Therefore, the within-village basic reproduction number was defined as follows: .
PPRV transmission between villages.
While the village component was deterministic, intervillage transmission was stochastic. Homogeneous mixing was assumed, with respect to the region of origin. At time t, the risk of having at least one susceptible small ruminant in a noninfected village i in region r becoming infected due to infected small ruminants in other villages was computed as follows: . A random number was generated between 0 and 1. If it was lower than , PPRV was introduced in village i, ; if not, . Note that, if , .
Parameter estimation.
All prior distributions were uniform with wide ranges (Table 1). The joint posterior distribution was estimated by repeated stochastic simulations using ABC-SMC. A simulation matched the data if distances between summary statistics computed for simulated and observed datasets were below given thresholds. The summary statistics were as follows: (i) the observed () and simulated () number of positive animals in region r, and (ii) the observed () and simulated () proportions of sampled kebeles in region r with an apparent seroprevalence falling within a range l: [0–5%[, [5–10%[, [10–20%[, [20–30%[, [30–40%[, [40–50%[, and [50–100%]. As there were two regions, there were thus four pairs of summary statistics, compared using the distance functions and . The latter was based on the relative entropy (55), following ref. 23. To ensure that this function was defined (i.e., all and ), 1 was added to every bin (i.e., to the number of observed and simulated kebeles falling in each of the seven seroprevalence ranges). The algorithm is further detailed in SI Appendix. Other parameters were fixed, and their values assessed based on a review of the literature (SI Appendix, Table S2). The impact of variations in these values on transmission parameter estimates was explored (SI Appendix).
Simulations and outcomes.
The duration of a simulation was drawn from a uniform distribution ranging from 20 to 25 y, as PPRV was assumed to have circulated in Ethiopia for at least 20 y before the 1999 survey (12, 13, 56). The infection was introduced at the start of a simulation, and every 500 d for the first 8 y of a simulation. Based on the posterior distribution, we computed the posterior predictive values of the village-level reproduction numbers , defined as the expected number of villages in region r infected by a single infected village in region k, in an initially fully susceptible metapopulation. It measured the potential of PPRV to be sustained within a region (k = r) and from a region to another (k ≠ r) (SI Appendix). The within-village and village-level immunity levels preventing PPRV transmission were assessed, as well as the annual vaccination coverage required to maintain immunity levels above these thresholds. The mathematical model was coded in the C language, and the ABC-SMC algorithm was implemented in R, version 3.2.2 (57). Scripts are available at https://bit.ly/2MxQD7d.
Supplementary Material
Acknowledgments
We thank Bryony A. Jones for her suggestions.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1711646115/-/DCSupplemental.
References
- 1.Albina E, et al. Peste des petits ruminants, the next eradicated animal disease? Vet Microbiol. 2013;165:38–44. doi: 10.1016/j.vetmic.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Barrett T, Pastoret PP, Taylor WP. Rinderpest and Peste des Petits Ruminants: Virus Plagues of Large and Small Ruminants. Academic; Cambridge, MA: 2005. [Google Scholar]
- 3.Goode E. February 14, 2017. The Saigas are struck again. NY Times, Section D, p 6.
- 4.Hoffmann B, et al. Fatalities in wild goats in Kurdistan associated with peste des petits ruminants virus. Transbound Emerg Dis. 2012;59:173–176. doi: 10.1111/j.1865-1682.2011.01270.x. [DOI] [PubMed] [Google Scholar]
- 5.Marashi M, et al. Peste des petits ruminants virus in vulnerable wild small ruminants, Iran, 2014–2016. Emerg Infect Dis. 2017;23:704–706. doi: 10.3201/eid2304.161218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahapatra M, et al. Spillover of peste des petits ruminants virus from domestic to wild ruminants in the Serengeti ecosystem, Tanzania. Emerg Infect Dis. 2015;21:2230–2234. doi: 10.3201/eid2112.150223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Food and Agriculture Organization of the United Nations–World Organisation for Animal Health 2015. Global Strategy for the Control and Eradication of PPR (Food and Agriculture Organization of the United Nations–World Organisation for Animal Health, Paris)
- 8.Kumar N, Barua S, Riyesh T, Tripathi BN. Advances in peste des petits ruminants vaccines. Vet Microbiol. 2017;206:91–101. doi: 10.1016/j.vetmic.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taye M, Deribe B, Melekot MH. Reproductive performance of central highland goats under traditional management in Sekota district, Ethiopia. Asian J Biol Sci. 2013;6:271–276. doi: 10.3923/pjbs.2013.692.696. [DOI] [PubMed] [Google Scholar]
- 10.Gizaw S, Tegegne A, Gebremedhin B, Hoekstra D. 2010. Sheep and goat production and marketing systems in Ethiopia: Characteristics and strategies for improvement (Nairobi, International Livestock Research Institute), Improving Productivity and Market Success of Ethiopian Farmers Project Working Paper 23.
- 11.FAO–Ethiopia . Strategy for Progressive Control of PPR in Ethiopia. Food and Agriculture Organization of the United Nations and Ethiopia; Addis Ababa, Ethiopia: 2012. [Google Scholar]
- 12.Roeder PL, Abraham G, Kenfe G, Barrett T. Peste des petits ruminants in Ethiopian goats. Trop Anim Health Prod. 1994;26:69–73. doi: 10.1007/BF02239901. [DOI] [PubMed] [Google Scholar]
- 13.Waret-Szkuta A, et al. Peste des petits ruminants (PPR) in Ethiopia: Analysis of a national serological survey. BMC Vet Res. 2008;4:34. doi: 10.1186/1746-6148-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keeling MJ, Rohani P. Modelling Infectious Diseases in Humans and Animals. Princeton Univ Press; Princeton: 2008. [Google Scholar]
- 15.Coppock DL. 1994. The Borana Plateau of southern Ethiopia: Synthesis of pastoral research, development and change, 1980–91 (International Livestock Centre for Africa, Addis Ababa, Ethiopia), Systems Study No. 5.
- 16.Baars RM. Costs and returns of camels, cattle and small ruminants in pastoral herds in eastern Ethiopia. Trop Anim Health Prod. 2000;32:113–126. doi: 10.1023/a:1005282719931. [DOI] [PubMed] [Google Scholar]
- 17.Devereux S. 2006. Vulnerable livelihoods in Somali Region, Ethiopia (Institute of Development Studies, Brighton, UK), IDS Research Report 57.
- 18.Getachew T, et al. Herd management and breeding practices of sheep owners in a mixed crop-livestock and a pastoral system of Ethiopia. Afr J Agric Res. 2010;5:685–691. [Google Scholar]
- 19.Jabbar M, Negassa A, Gidyelew T. 2007. Geographic distribution of cattle and shoats populations and their market supply sheds in Ethiopia (International Livestock Research Institute, Nairobi), ILRI Improving Market Opportunities Discussion Paper No. 2.
- 20.Gebremedhin B, Hoekstra D, Jemaneh S. 2007. Heading towards commercialization? The case of live animal marketing in Ethiopia (International Livestock Research Institute, Nairobi), Improving Productivity and Market Success of Ethiopian Farmers Project Working Paper 5.
- 21.Toni T, Welch D, Strelkowa N, Ipsen A, Stumpf MP. Approximate Bayesian computation scheme for parameter inference and model selection in dynamical systems. J R Soc Interface. 2009;6:187–202. doi: 10.1098/rsif.2008.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooks-Pollock E, Roberts GO, Keeling MJ. A dynamic model of bovine tuberculosis spread and control in Great Britain. Nature. 2014;511:228–231. doi: 10.1038/nature13529. [DOI] [PubMed] [Google Scholar]
- 23.Conlan AJ, et al. Estimating the hidden burden of bovine tuberculosis in Great Britain. PLoS Comput Biol. 2012;8:e1002730. doi: 10.1371/journal.pcbi.1002730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gubbins S, et al. Inferences about the transmission of Schmallenberg virus within and between farms. Prev Vet Med. 2014;116:380–390. doi: 10.1016/j.prevetmed.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beyene TJ, Abegaz BA, Chibsa TR. Trend analysis of herd composition and its trade implication in Borena, southern pastoral area of Ethiopia. Livest Res Rural Dev. 2013;25:47. [Google Scholar]
- 26.Faris D, Yilkal A, Berhe G, Kelay B. Seroprevalence and sero-conversion after vaccination against peste des petits ruminants in sheep and goats from Awash Fentale district, Afar, Ethiopia. Prev Vet Med. 2012;103:157–162. doi: 10.1016/j.prevetmed.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Mariner JC, Gachanja J, Tindih SH, Toye P. A thermostable presentation of the live, attenuated peste des petits ruminants vaccine in use in Africa and Asia. Vaccine. 2017;35:3773–3779. doi: 10.1016/j.vaccine.2017.05.040. [DOI] [PubMed] [Google Scholar]
- 28.Fournié G, et al. Investigating poultry trade patterns to guide avian influenza surveillance and control: A case study in Vietnam. Sci Rep. 2016;6:29463. doi: 10.1038/srep29463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parida S, et al. Peste des petits ruminants. Vet Microbiol. 2015;181:90–106. doi: 10.1016/j.vetmic.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abraham G, et al. Antibody seroprevalences against peste des petits ruminants (PPR) virus in camels, cattle, goats and sheep in Ethiopia. Prev Vet Med. 2005;70:51–57. doi: 10.1016/j.prevetmed.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 31.Megersa B, et al. Serological investigation of peste des petits ruminants (PPR) in small ruminants managed under pastoral and agro-pastoral systems in Ethiopia. Small Rumin Res. 2011;97:134–138. [Google Scholar]
- 32.Muniraju M, et al. Emergence of lineage IV peste des petits ruminants virus in Ethiopia: Complete genome sequence of an Ethiopian isolate 2010. Transbound Emerg Dis. 2016;63:435–442. doi: 10.1111/tbed.12287. [DOI] [PubMed] [Google Scholar]
- 33.Libeau G, Diallo A, Parida S. Evolutionary genetics underlying the spread of peste des petits ruminants virus. Anim Front. 2014;4:14–20. [Google Scholar]
- 34.Kivaria FM, et al. The incursion, persistence and spread of peste des petits ruminants in Tanzania: Epidemiological patterns and predictions. Onderstepoort J Vet Res. 2013;80:593. doi: 10.4102/ojvr.v80i1.593. [DOI] [PubMed] [Google Scholar]
- 35.EFSA Panel on Animal Health and Welfare Scientific opinion on peste des petits ruminants. EFSA J. 2015;13:3985. [Google Scholar]
- 36.Intisar KS, et al. Peste des petits ruminants infection in domestic ruminants in Sudan. Trop Anim Health Prod. 2017;49:747–754. doi: 10.1007/s11250-017-1254-3. [DOI] [PubMed] [Google Scholar]
- 37.Kihu SM, et al. Sero-epidemiology of peste des petits ruminants virus infection in Turkana County, Kenya. BMC Vet Res. 2015;11:87. doi: 10.1186/s12917-015-0401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kardjadj M, Kouidri B, Metref D, Luka PD, Ben-Mahdi MH. Seroprevalence, distribution and risk factor for peste des petits ruminants (PPR) in Algeria. Prev Vet Med. 2015;122:205–210. doi: 10.1016/j.prevetmed.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Rony MS, Rahman AK, Alam MM, Dhand N, Ward MP. Peste des petits ruminants risk factors and space-time clusters in Mymensingh, Bangladesh. Transbound Emerg Dis. 2017;64:2042–2048. doi: 10.1111/tbed.12615. [DOI] [PubMed] [Google Scholar]
- 40.Munir M. Role of wild small ruminants in the epidemiology of peste des petits ruminants. Transbound Emerg Dis. 2014;61:411–424. doi: 10.1111/tbed.12052. [DOI] [PubMed] [Google Scholar]
- 41.Roger F, et al. Investigation of a new pathological condition of camels in Ethiopia. J Camel Pract Res. 2000;2:163–166. [Google Scholar]
- 42.Jones BA, et al. The economic impact of eradicating peste des petits ruminants: A benefit-cost analysis. PLoS One. 2016;11:e0149982. doi: 10.1371/journal.pone.0149982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molia S, et al. Live bird markets characterization and trading network analysis in Mali: Implications for the surveillance and control of avian influenza and Newcastle disease. Acta Trop. 2016;155:77–88. doi: 10.1016/j.actatropica.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Mweu MM, Fournié G, Halasa T, Toft N, Nielsen SS. Temporal characterisation of the network of Danish cattle movements and its implication for disease control: 2000–2009. Prev Vet Med. 2013;110:379–387. doi: 10.1016/j.prevetmed.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 45.Ortiz-Pelaez A, Ashenafi G, Roger F, Waret-Szkuta A. Can geographical factors determine the choices of farmers in the Ethiopian highlands to trade in livestock markets? PLoS One. 2012;7:e30710. doi: 10.1371/journal.pone.0030710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dezso Z, Barabási AL. Halting viruses in scale-free networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2002;65:055103. doi: 10.1103/PhysRevE.65.055103. [DOI] [PubMed] [Google Scholar]
- 47.Hammami P, Lancelot R, Lesnoff M. Modelling the dynamics of post-vaccination immunity rate in a population of Sahelian sheep after a vaccination campaign against peste des petits ruminants virus. PLoS One. 2016;11:e0161769. doi: 10.1371/journal.pone.0161769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dean AS, et al. Potential risk of regional disease spread in West Africa through cross-border cattle trade. PLoS One. 2013;8:e75570. doi: 10.1371/journal.pone.0075570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baudon E, et al. Analysis of swine movements in a province in Northern Vietnam and application in the design of surveillance strategies for infectious diseases. Transbound Emerg Dis. 2017;64:411–424. doi: 10.1111/tbed.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Central Statistical Authority 2012. 2007 Population and Housing Census of Ethiopia (Central Statistical Authority, Addis Ababa, Ethiopia)
- 51.Pantuliano S, Wekesa M. Improving Drought Response in Pastoral Areas of Ethiopia. (Humanitarian Policy Group Overseas Development Institute; London: 2008. [Google Scholar]
- 52.Behnke R. 2010. Contribution of livestock to the economies of IGAD member states study findings, application of the methodology in Ethiopia and recommendations for further work (Intergovernmental Authority on Development, Addis Ababa, Ethiopia), IGAD LPI Working Paper No. 02–10.
- 53.Central Statistical Authority 2003. Ethiopia—2001–2002 Agricultural Sample Enumeration (Central Statistical Authority, Addis Ababa, Ethiopia)
- 54.Abebe Y, Melaku S, Tegegne A. Sheep breeds, traditional breeding and flock structure in Burie district, North Western Ethiopia. Global Adv Res J Agric Sci. 2013;2:325–335. [Google Scholar]
- 55.Kullback S, Leibler RA. On information and sufficiency. Ann Math Stat. 1951;22:79–86. [Google Scholar]
- 56.Pegram RG, Tereke F. Observation on the health of Afar livestock. Ethiop Vet J. 1981;5:11–14. [Google Scholar]
- 57.R Core Team 2015. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna), R 3.2.2.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.