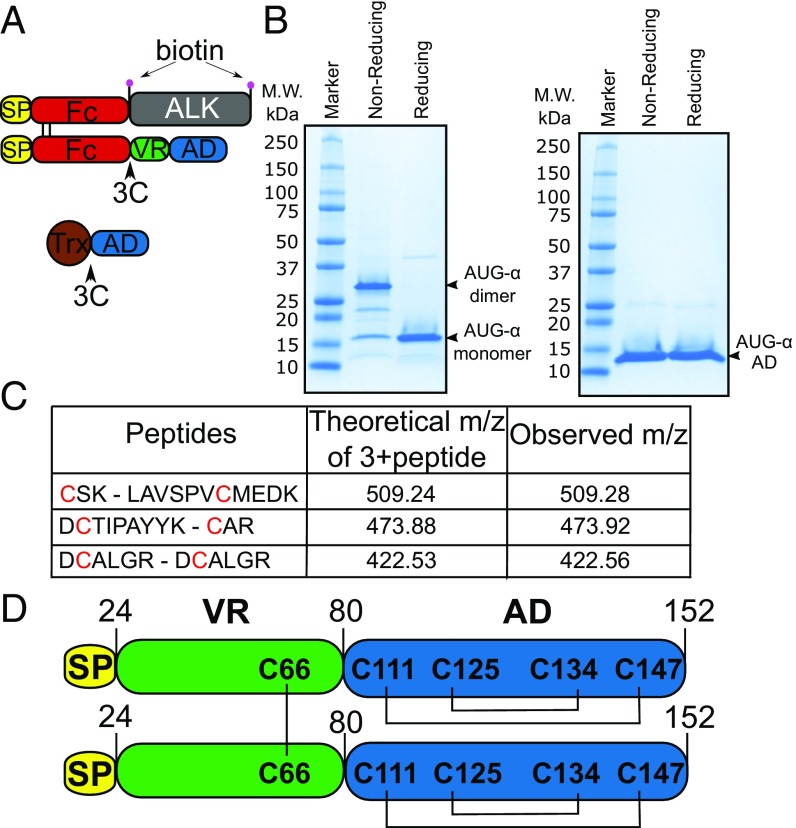
Fig. 1.
AUG-α is expressed as a disulfide bridged dimer. (A) Schematic representation of expression constructs to produce full-length AUG-α from mammalian cells (Upper) or AUG-α AD from bacteria (Lower). Signal peptide is colored in yellow, Fc fragment in red, ALK fragment (residues 648–1030) in gray, AUG-α variable region (VR) in green, augmentor domain (AD) in blue, and Trx in brown. The 3C protease cleavage site is marked by an arrow, and the biotin acceptor peptides are marked by pink circles. (B) SDS/PAGE analysis of purified full-length AUG-α (Left) and a truncated version, AUG-α AD (Right), separated under nonreducing and reducing conditions as indicated in the upper panels. Molecular weight (M.W.) marker with corresponding molecular masses is shown on the left side of the gels. Under nonreducing conditions, full-length AUG-α migrates as a dimer, but migrates as a monomer upon reduction (as indicated on the right side). (C) Mass spectrometry mapping of disulfide bridges in AUG-α. Purified AUG-α was subjected to nonreducing SDS/PAGE, and the band corresponding to the dimeric form of AUG-α was excised, digested with trypsin, and used for mass spectrometry analysis. Disulfide-linked peptides are presented in the first column, and corresponding cysteines are highlighted in red. Theoretically predicted molecular masses for the corresponding 3+peptides are listed in the second column, and the observed m/z is listed in the last column. (D) Schematic representation of full-length AUG-α. Color code is the same as in A and B. Disulfide bridges are shown by black lines.