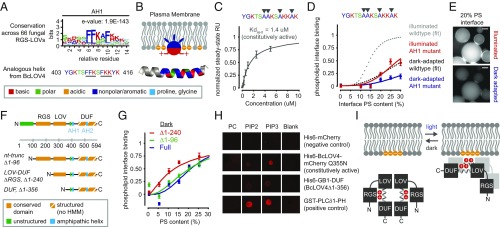
Fig. 4.
Structure–function determinants of the BcLOV4–lipid interaction. (A) Sequence logo of amphipathic helix (AH1) conserved among 66 RGS-LOV homologs (Top) and the specific sequence for BcLOV4, which includes known lipid binding motifs (underlined) (Bottom). (B) Schematized membrane insertion mechanism of the AH1, where hydrophobic residues embed into the hydrophobic bilayer and basic residues electrostatically bind anionic phospholipids. (C) SPR-determined affinity of AH1 mutant for 20% PS bilayers, with hydrophobic residues mutated to alanine, is reduced ∼10-fold from wild-type BcLOV4. SPR data are of constitutively active Q355N mutant (n = 2; error, SD). (D) Phospholipid interface binding curves, calculated as the membrane interface/dispersed phase ratio (normalized) of the AH1 mutant (no Q355N) when dark-adapted or illuminated with blue light in w/o emulsions, normalized to wild-type saturation level under illumination (n = 20–200 droplets; error, SEM). Dotted fits for perspective derived from Fig. 3. (E) Representative fluorescence micrographs showing that the AH1 mutant primarily remains in the aqueous dispersed phase upon illumination. (Scale bar: 25 μm.) (F) Truncations created to probe domain contributions to light-switched membrane association. HMM, existing hidden Markov model. (G) Phospholipid interface binding curves, calculated as the membrane interface/dispersed phase ratio (normalized) of BcLOV4, nt-truncated protein, and RGS-truncated LOV-DUF, in w/o emulsions and in the absence of illumination. Increased binding by deletion of the RGS suggests that the RGS domain inhibits the membrane interaction in the absence of illumination. Normalized to wild-type saturation level under illumination. n = 30–125 droplets; error, SEM. (H) Lipid-blot assays for DUF–lipid interaction (3-nmol lipid per spot). Visualized with IRDye680-conjugated anti-mouse IgG secondary antibody against primary mouse anti-His6 or anti-GST antibodies. The DUF bound negatively charged phosphoinositides, but not zwitterionic PC. (I) Schematized proposed signal transmission mode of BcLOV4, with the oligomer drawn as a dimer for clarity.