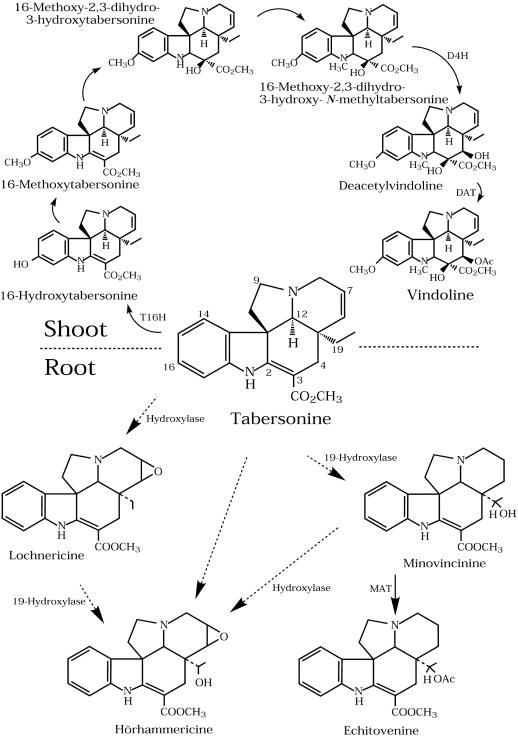
Figure 1.
Biosynthesis of tabersonine-derived indole alkaloids in Madagascar periwinkle organs. Tabersonine is converted into vindoline via six enzymatic steps, with the terminal hydroxylation (D4H) and O-acetylation (DAT) occurring in specialized cells known as idioblasts and laticifers of leaves and stems [1]. Tabersonine is converted into lochnericine, hörhammericine, and minovincinine via uncharacterized hydroxylations and 19-hydroxy-indole alkaloids are converted into their respective products by O-acetylation (MAT). These tabersonine analogs are known to accumulate under certain conditions within cell cultures (Kutney et al., 1980) and roots (Shanks et al., 1998) of Madagascar periwinkle.