Abstract
This study used the 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide radical (PTIO•) trapping model to study the antioxidant activities of 16 natural xanthones in aqueous solution, including garcinone C, γ-mangostin, subelliptenone G, mangiferin, 1,6,7-trihydroxy-xanthone, 1,2,5-trihydroxyxanthone, 1,5,6-trihydroxyxanthone, norathyriol, 1,3,5,6-tetrahydroxy-xanthone, isojacareubin, 1,3,5,8-tetrahydroxyxanthone, isomangiferin, 2-hydroxyxanthone, 7-O-methylmangiferin, neomangiferin, and lancerin. It was observed that most of the 16 xanthones could scavenge the PTIO• radical in a dose-dependent manner at pH 4.5 and 7.4. Among them, 12 xanthones of the para-di-OHs (or ortho-di-OHs) type always exhibited lower half maximal inhibitory concentration (IC50) values than those not of the para-di-OHs (or ortho-di-OHs) type. Ultra-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry (UPLC-ESI-Q-TOF-MS/MS) analysis revealed that most of these xanthones gave xanthone-xanthone dimers after incubation with PTIO•, except for neomangiferin. Based on these data, we concluded that the antioxidant activity of phenolic xanthone may be mediated by electron-transfer (ET) plus H+-transfer mechanisms. Through these mechanisms, some xanthones can further dimerize unless they bear huge substituents with steric hindrance. Four substituent types (i.e., para-di-OHs, 5,6-di-OHs, 6,7-di-OHs, and 7,8-di-OHs) dominate the antioxidant activity of phenolic xanthones, while other substituents (including isoprenyl and 3-hydroxy-3-methylbutyl substituents) play a minor role as long as they do not break the above four types.
Keywords: xanthone, structure-activity relationship, antioxidant, ortho-di-OHs, para-di-OHs
1. Introduction
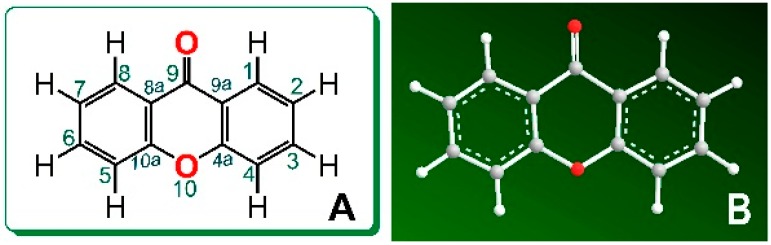
Natural xanthones can be isolated from edible plants, medicinal plants (including Chinese herbal medicines [1]), and marine-derived fungi (e.g., Talaromyces islandicus EN-501 [2]). Particularly, dozens of xanthones have been successfully isolated from the tropical fruits mangosteen and mango, and xanthones have been considered as the bioactive constituents of these fruits [3,4,5,6]. Structural elucidation demonstrated that the xanthone scaffold is composed of two phenyl rings and one pyrone ring in the same plane (Figure 1). In this planar and symmetrical scaffold, the hydrogen atom (H) at the 1–8 position can be substituted by -OH to construct phenolic -OHs; thus, xanthones can be regarded as natural phenolics and serve as phenolic antioxidants [3,4,7].
Figure 1.
The structure (A) and preferential conformation (B) of the xanthone scaffold.
Phenolic antioxidants were reported to play an important role in disease prevention [5]. For example, mangosteen fruit was recently suggested to have an anti-tumor effect towards hepatic carcinoma [8]. Indeed, anti-tumor activity is closely associated with antioxidant activity. This is because cell carcinogenesis results—to a great extent—from reactive oxygen species (ROS)-induced oxidative damage [9]. Phenolic xanthones and other phenolics can effectively suppress the excessive ROS to prevent carcinogenesis. Nevertheless, a systematic investigation of the antioxidant activities and mechanisms of the xanthones family has not yet been reported.
In the study, a 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide radical (PTIO•, Figure 2) trapping assay was introduced for the investigation. The PTIO•-trapping model has been newly developed by our team [10], and has at least two advantages over the common antioxidant assays, e.g., 1,1-diphenyl-2-picryl-hydrazl (DPPH•) scavenging assay and 2,2′-azino-bis(3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS•+) scavenging assay. Like the •OH radical, the •O2- anion radical, and other ROS, the PTIO• radical is also an oxygen-centered radical (Figure 2); Like antioxidant action in cells, PTIO• trapping action is also fulfilled in aqueous media; aqueous media however is more biologically relevant [11,12]. Two common antioxidant assays (especially the DPPH•-scavenging assay), however, are based on a nitrogen-centered radical and performed in lipophilic media. Thus, the PTIO•-trapping assay can well characterize the ROS-scavenging action of an antioxidant and is more suitable to study the antioxidant activity of phenolic xanthones. To research their antioxidant mechanisms, the reaction products of xanthones with PTIO• were further determined using ultra-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry (UPLC−ESI−Q−TOF−MS/MS) in the study.
Figure 2.
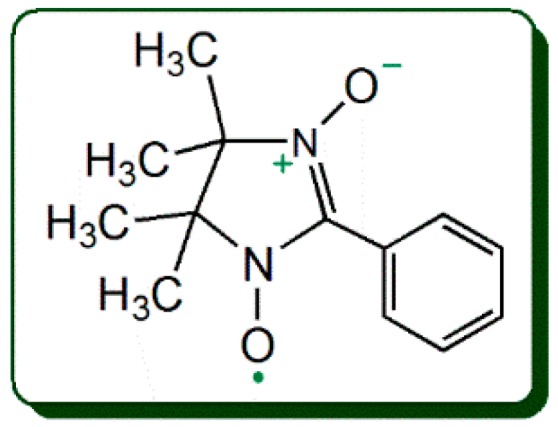
The structure of the 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide radical (PTIO•).
Hypothetically, the antioxidant activities and mechanisms should arise from the effectiveness of some specific substituents in xanthones. As indicated in the literature [13,14], these substituents mainly refer to phenolic -OH, isoprenyl, cyclized-isoprenyl, methyl, and glycoside. Phenolic -OH, however, can be further classified into several types: para-di-OHs, 5,6-di-OHs, 6,7-di-OHs, 7,8-di-OHs, single phenolic -OH, and meta-di-OHs. The isoprenyl substituent is considered as a characteristic of the xanthones family, because about 50% members contain this substituent [13,14]. Whether and how these substituents affect the antioxidant activities of xanthones remain unknown until now, despite the fact that at least 300 phenolic xanthones have already been recorded in the literature [13,14].
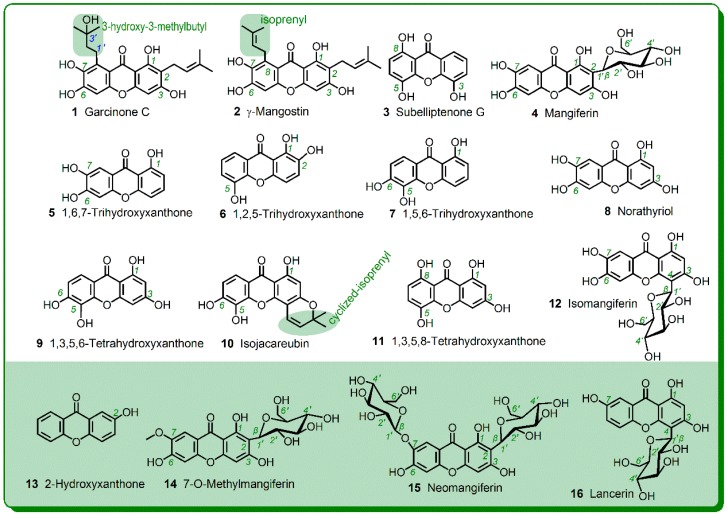
To address the above problems, 16 xanthones were randomly selected as references in this study (Figure 3). As seen in Figure 3, these references covered all the aforementioned substituents. Moreover, they account for about 6% of whole xanthones [13,14]. Thereby, the study is expected to provide new and characteristic information of phenolic xanthones.
Figure 3.
Structures of the 16 selected xanthones.
2. Results and Discussion
2.1. Antioxidant Activity and Mechanism in PTIO•-Trapping Assay
As suggested by the cyclic voltammogram, PTIO•-trapping below pH 5.0 is an ET process [10]. In the present study, most of xanthones could scavenge the PTIO• radical in a dose-dependent manner at pH 4.5 (Supplementary Materials S1). This indicated that xanthones might possess ET potential during the antioxidant process. Furthermore, the PTIO•-trapping activities of these xanthones were also determined at physiological pH (7.4). PTIO•-trapping action at pH 7.4 may be involved in H+-transfer [10]. As shown in Supplementary Materials S1, the PTIO•-trapping percentages of the 16 xanthone references also increased in a concentration-dependent manner at pH 7.4, implying that the antioxidant activity of xanthone may also be involved in H+-transfer. In fact, at physiological pH, phenolic -OH with weak acidity may ionize to give rise to H+. For example, mangiferin has been demonstrated to be of pKa1 6.52 ± 0.006 (25 °C) [15]. At pH 4.5, its weak acidity may be suppressed by the solution H+ ion, thus its H+-transfer has been reduced. Accordingly, it exhibited higher IC50 value at pH 4.5, compared with at pH 7.4 (Table 1).
Table 1.
The main results of PTIO•-trapping activity of 16 xanthones using UPLC-ESI-Q-TOF-MS/MS analysis and colorimetric analysis.
| No | Xanthone | Main Results of UPLC-ESI-Q-TOF-MS/MS Analysis of Reaction Products | IC50 in Colorimetric Analysis/μM | Activity | ||||
|---|---|---|---|---|---|---|---|---|
| Retention Time/Min | Primary MS Spectra | MS/MS Spectra | Product | pH 4.5 | pH 7.4 | |||
| 1 | Garcinone C | 5.961 | 825, 826, 827 | 353, 411, 825 | dimer | 36.0 ± 3.1 | 40.8 ± 2.0 | Strong |
| 2 | γ-Mangostin | 9.847 | 789, 790, 791 | 375, 393, 394, 395, 677 | dimer | 45.5 ± 2.4 | 60.4 ± 1.8 | |
| 3 | Subelliptenone G | 1.739 | 487, 488 | No data | dimer | 63.4 ± 13.3 | 110.9 ± 3.8 | |
| 4 | Mangiferin | 1.105 | 841, 842, 843, 844 | 329, 419, 601, 631, 661, 721,751, 823 | dimer | 64.1 ± 8.5 | 38.0 ± 2.7 | |
| 5 | 1,6,7-Trihydroxyxanthone | 1.813 | 485, 846, 487 | 243, 349 | dimer | 83.0 ± 2.2 | 90.3 ± 3.0 | |
| 6 | 1,2,5-Trihydroxyxanthone | 1.648 | 487, 488 | No data | dimer | 89.1 ± 7.4 | 112.2 ± 0.2 | |
| 7 | 1,5,6-Trihydroxyxanthone | 2.044 | 485, 486, 487, 488 | 243, 485 | dimer | 101.3 ± 16.6 | 116.3 ± 2.2 | |
| 8 | Norathyriol | 1.341 | 517, 518, 519, 520 | 229, 257, 258, 259, 365, 499, 517 | dimer | 103.0 ± 3.7 | 54.1 ± 0.9 | |
| 9 | 1,3,5,6-Tetrahydroxyxanthone | 1.349 | 517, 518, 519, 520 | 229, 257, 258, 259, 365, 499, 517 | dimer | 108.1 ± 19.4 | 102.7 ± 4.7 | |
| 10 | Isojacareubin | 8.483 | 649, 650, 651, 652 | 323-325, 649 | dimer | 108.7 ± 0.1 | 136.7 ± 7.3 | |
| 11 | 1,3,5,8-Tetrahydroxyxanthone | 1.726 | 519, 520, 521 | 215, 259, 260 | dimer | 116.7 ± 12.6 | 133.1 ± 29.4 | |
| 12 | Isomangiferin | 1.105 | 841, 842, 843, 844 | 329, 419, 601, 631, 661, 721,751, 823 | dimer | 121.3 ± 8.3 | 104.1 ± 12.3 | |
| 13 | 2-Hydroxyxanthone | 1.885 | 423, 424 | No data | dimer | 284.2 ± 48.8 | 142.5 ± 13.1 | Weak |
| 14 | 7-O-Methylmangiferin | 1.204 | 871, 872 | No data | dimer | 387.2 ± 37.5 | 234.9 ± 1.7 | |
| 15 | Neomangiferin | No product | No product | No product | No product | 545.2 ± 15.2 | 213.1 ± 23.8 | |
| 16 | Lancerin | 1.165–1.191 | 811, 812 | No date | dimer | 681.2 ± 7.9 | 1106.3 ± 202.6 | |
The IC50 value was defined as the final concentration of 50% PTIO• radical inhibition and was calculated by linear regression analysis and expressed as the mean ± SD (n = 3). The linear regression was analyzed using Origin 6.0 professional software. Trolox is the positive control. Its IC50 values were calculated as 187.5 ± 24.2 μM (pH 4.5) and 175.0 ± 12.4 μM (pH 7.4). The dose-response curves are listed in Supplementary Materials S1; while the original UPLC-ESI-Q-TOF-MS/MS data were listed in Supplementary Materials S18. The 16 xanthones were classified based on the comparison of IC50 values at pH 4.5. These data were analyzed by independent t-test for comparison between two groups. Multiple comparisons within the same group was conducted by one-way ANOVA. p < 0.05 was considered statistically significant. The statistical analysis was performed using SPSS 11.5 system (SPSS, Chicago, IL, USA).
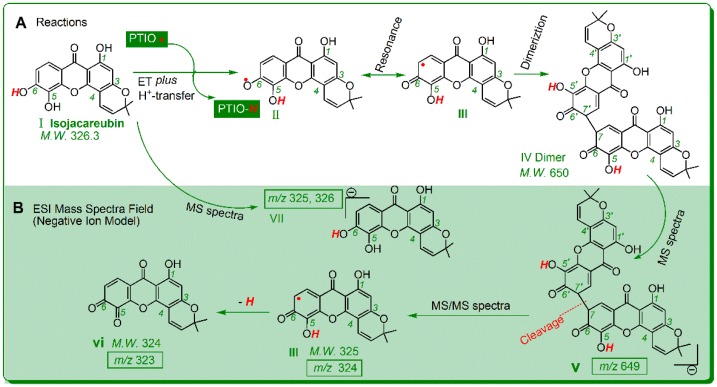
To study the antioxidant mechanisms further, each of xanthones was incubated with the PTIO• radical and the reaction product was subsequently analyzed using UPLC−ESI−Q−TOF−MS/MS. The results in Table 1 revealed that most of these xanthones gave rise to a dimeric product. For example, after incubation with the PTIO• radical, isojacareubin yielded an isojacareubin-isojacareubin dimer, which displayed a primary MS peak (m/z 649) under negative ion model. This primary MS peak was further observed to cleave to produce m/z 323 and 324 (Table 1 and Supplementary Materials S18). Based on these MS spectral data and previous work [16], the dimerization reaction of isojacareubin can be proposed as Figure 4A; while the MS spectra elucidation is described in Figure 4B.
Figure 4.
The possible reactions of isojacareubin with PTIO• (A), and the MS spectra elucidations (B) (The m/z value is expressed as an integer; precise m/z values are detailed in Supplementary Materials S18.).
In the proposed reactions (Figure 4), the covalent bond between the 7- and 7′- positions has linked two isojacareubin radicals (III), to generate one isojacareubin-isojacareubin dimer (IV). The generation of a dimer can be considered as the consequence of ET plus H+-transfer (Figure 4). If there is no H+-transfer, there will be no peak of m/z 649 in the product. On the other hand, if there is only H+-transfer and no ET, there will be a phenoxy anion; two phenoxy anions however cannot combine with each other via covalency. Therefore, the evidence from UPLC−ESI−Q−TOF−MS/MS analysis can further verify the occurrence of ET plus H+-transfer.
It is worth mentioning that, (i) the dimerization reaction may be more complicated than that in Figure 4, and the covalent bond can also be linked to other positions [17,18,19]. Nevertheless, it is doubtless that the dimeric isojacareubin-isojacareubin is formed. (ii) The only xanthone that did not give a dimer product was neomangiferin (Table 1) bearing two sugar residues. This can be attributed to the fact that the two sugar residues have huge substituents and may hinder the radical adduct formation (RAF) potential. The RAF product however was supported by the earlier literatures where it was termed as nonradical product [11,20,21].
2.2. Antioxidant Role of Phenolic -OH: Evidence From PTIO•-Trapping Assay
Results from the colorimetric method revealed that there was a great difference in the H+-transfer (or ET) potentials among the 16 references (Table 1). At pH 4.5, the values of IC50 varied from 36.0 μM to 681.2 μM. Particularly, there was an evident gap in IC50 values at pH 4.5 between the former twelve xanthones (1–12, IC50 = 36.0–121.3 μM, pH = 4.5) and the latter four xanthones (13–16, IC50 = 284.2–681.2 μM, pH = 4.5, Table 1). The latter four xanthones contained neither para-di-OHs nor ortho-di-OHs. In this case, even with multiple phenolic -OHs, the xanthone still exhibited very low high IC50 values, e.g., neomangiferin (15). The data of neomangiferin clearly indicated that, para-di-OHs (or ortho-di-OHs) played a critical role in antioxidant activity at pH 4.5; and single phenolic -OHs and meta-di-OHs type played a negligible role. As seen in Table 1, both para-di-OHs and ortho-di-OHs xanthones are classified into the strong group based on the IC50 values at pH 4.5, implying that the role of para-di-OHs is roughly equivalent to that of ortho-di-OHs. A typical example was the pair of 9 vs. 11 at pH 4.5; A similar situation was also observed at pH 7.4. At pH 7.4, however, the PTIO•-trapping has been mentioned to be mediated by H+-transfer [10]. Thus, it can be inferred that para-di-OHs or ortho-di-OHs may similarly govern the ET plus H+-transfer potentials.
Further analysis suggested that ortho-di-OHs could be divided into 3 types, i.e., 5,6-di-OHs (e.g., 7, 9, and 10), 6,7-di-OHs (e.g., 1, 2, 4, 5, 8, and 12), and 7,8-di-OHs (e.g., 6). The fact that the IC50 values of 3 xanthones (1,6,7-trihydroxyxanthone (5), 1,2,5-trihydroxyxanthone (6), and 1,5,6-trihydroxyxanthone (7)) are not significantly different (p > 0.05) at pH 4.5 suggests that the ortho-di-OHs positions (5,6-position, 6,7- position, or 7,8-position) have not affected the antioxidant activity. This suggestion can partly explain the similarities of a pair of xanthones: norathyriol (8) and its isomer 1,3,5,6-tetrahydroxyxanthone (9). As shown in Table 1, in PTIO•-trapping colorimetric analysis, there is no significant difference (p > 0.05) in IC50 values at pH 4.5 between them. In PTIO•-trapping UPLC−ESI−Q−TOF−MS/MS analysis, norathyriol and its isomer produced a similar series of MS peaks (Table 1 and Supplementary Materials S18). Based on the MS spectra elucidation, it is presumed that these two isomers have undergone similar dimerization reactions (Supplementary Materials S19,20).
In short, during the PTIO•-trapping process, meta-di-OHs have a negligible effect on the occurrence of ET plus H+-transfer reactions, and each of the other four substituent types (para-di-OHs, 5,6-di-OHs, 6,7-di-OHs, and 7,8-di-OHs) can govern the antioxidant activity of phenolic xanthones. However, if any of the four types is broken, the antioxidant activity is remarkedly decreased. For example, when 6,7-di-OHs in mangiferin (4) were broken by a glucoside to form neomangiferin (15), the IC50 values were greatly increased (8.5-times for pH 4.5 and 5.6-times for pH 7.5, Table 1).
Our findings regarding the role of ortho-di-OHs was further supported by a number of reports, which usually referred to ortho-di-OHs as the catechol moiety [22,23,24,25,26]. The reason why the ortho-di-OHs play a critical role in antioxidant xanthones may be the fact that ortho-di-OHs can be oxidized to ortho-benzo quinone by free radicals [27,28]. For example, in the reaction of isojacareubin with PTIO•, 5,6-di-OHs is oxidized to 5,6-ortho-benzoquinone via ET plus H+-transfer mechanisms [29,30] (Figure 4). Similarly, para-di-OHs type could be hypothesized to transform into para-benzoquinone, in accordance with Figure 4. In fact, the para-di-OHs molecule itself has strong antioxidant activity. It is now clear that the stability of para-benzoquinone or ortho-benzoquinone can be responsible for the strong antioxidant activity of para-di-OHs (or ortho-di-OHs) type. Our experimental data and deduction denied the opinion of a minor role of ortho-di-OHs type (catechol moiety) [31].
It must be emphasized that (1) the antioxidant role of para-di-OHs type in natural phenolics has not been mentioned previously [32]. This may be attributed to the fact that para-di-OHs are hardly found in flavonoids [13,14,26,33] and other phenolics [13,14,19,22,23,24,25,26,27,28,29,30,31,34,35]. For instance, among thousands of flavonoids, only 5 para-di-OHs flavonoids (i.e., 5,8-dihydroxy-flavonoids) have been documented: rhodionin [36], 5,8-dihydroxy-3,6,7-trimethoxyflavone [37], 5,8-dihydroxy-6,7-dimethoxyflavone [37], 5,8-dihydroxy-6,7,4′-trimethoxyflavone, and 5,8-dihydroxy-6,7,3′,4′,5′-pentamethoxyflavone [38]. Thereby, our findings may be of great scientific value. (2) ET plus H+-transfer reactions can cover several possible mechanisms, such as hydrogen atom transfer (HAT), sequential proton-loss electron-transfer (SPLET), electron transfer−proton transfer (ET−PT), and proton coupled electron transfer (PCET) [35,39]. Because these antioxidant mechanisms are essentially involved in ET and H+-transfer reactions. The difference among these mechanisms depends on the sequence and cooperativity [40]. Thus, the net result of all these different antioxidant mechanisms is identical; and the proposed reactions in Figure 4 are basically acceptable. Our proposal is also supported by the recent report concerning phenolic antioxidant reaction with alkylperoxyl radical (ROO•) [41].
2.3. Antioxidant Role of Other Substituents: Evidence from the PTIO•-Trapping Assay
As discussed above, other substituents may also affect the antioxidant activity of xanthones. These substituents include isoprenyl, cyclized-isoprenyl, methyl, and glycoside. The isoprenyl substituent frequently appears in xanthones, and seldom occurs in other phenolics (such as flavonoid, lignanoid, coumarin, and stilbene [13,14,23,25,42,43,44,45,46]). As a result, its role has not been analyzed by means of the structure-activity relationship [40]. In the study, γ-mangostin (2), an isoprenylated xanthone was found to be superior to its parent compound norathyriol (8) at pH 4.5.
This suggested that the isoprenyl substituent enhances the ET potential. Possibly through hydrolysis, isoprenyl substituent can also be transferred into 3-hydroxy-3-methylbutyl substituent. Thus, 3-hydroxy-3-methylbutyl substituent can also be found in xanthones (e.g., 1, Figure 3). As shown in Table 1, there was no great difference between garcinone C (1) and γ-mangostin (2); Thus, the effect of the 3-hydroxy-3-methylbutyl substituent was basically equivalent to that of the isoprenyl substituent. In addition, the cyclized-isoprenyl substituent was also found in xanthone (e.g., 10, Figure 3). Comparison of the IC50 values of 1,3,5,6-tetrahydroxyxanthone (9) and isojacareubin (10) revealed that the cyclized-isoprenyl substituent decreases the antioxidant activity (Table 1). This may be attributed to the fact that the cyclized-isoprenyl substituent replaces a phenolic -OH at the 3-position.
Like the cyclized-isoprenyl substituent, the methyl substituent may also replace phenolic -OHs. A typical example was mangiferin (4) and its ether 7-O-methylmangiferin (14). As shown in Table 1, the IC50 values at two pH values (pH 4.5 and 7.4) decrease significantly from mangiferin (4) to 7-O-methylmangiferin (14). This is because methylation breaks the 6,7-di-OHs construction in mangiferin (4).
Like the methyl substituent, the glycoside substituent frequently occurs in phenolic antioxidants (including xanthones). As seen in Table 1, both mangiferin (4) and isomangiferin (12) can be regarded as the glycosidated derivatives of norathyriol (8); and mangiferin (4) and isomangiferin (12) are actually two positional-isomers of each other. However, in the study, the three xanthones were classified into as the strong antioxidants (Table 1). The fact implies that, the effect of glycoside substituent at any C-position is very limited.
In brief, during the PTIO•-trapping process, the above substituents played a minor role in antioxidant activity. However, when these substituents break the aforementioned four types (para-di-OHs, 5,6-di-OHs, 6,7-di-OHs, and 7,8-di-OHs), they can greatly lower the antioxidant activity of xanthones.
3. Materials and Methods
3.1. Chemicals and Animals
2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide radical (PTIO•, CAS 18390-00-6, >98.0%, M.W. 233.29) was purchased from TCI Chemical Co. (Shanghai, China). (±)-6-Hydroxyl-2,5,7,8-tetramethlychromane-2-carboxylic acid (Trolox, CAS 53188-07-1, 97%, M.W. 250.29) was purchased from Sigma-Aldrich Shanghai Trading Co. (Shanghai, China). Garcinone C (CAS 76996-27-5, C23H26O7, M.W. 414.5, 98%, Supplementary Materials S2) and γ-mangostin (CAS 31271-07-5, C23H24O6, M.W. 396.4, 97%, Supplementary Materials S3) were purchased from Chengdu Biopurify Phytochemicals Ltd. (Chengdu, China). Subelliptenone G (CAS 162473-22-5, C13H8O5, M.W. 244.2, purity 97%, Supplementary Materials S4) was purchased from BioBioPha Co., Ltd. (Kunming, China). Mangiferin (CAS 4773-96-0, C19H18O11, M.W. 422.3, 98%, Supplementary Materials S5) was purchased from Chengdu Biopurify Phytochemicals Ltd. 1,6,7-Trihydroxyxanthone (CAS 25577-04-2, C13H8O5, M.W. 244.2, purity 98%, Supplementary Materials S6), 1,2,5-trihydroxyxanthone (CAS 156640-23-2, C13H8O5, M.W. 244.2, purity 98%, Supplementary Materials S7), 1,5,6-trihydroxyxanthone (CAS 5042-03-5, C13H8O5, M.W. 244.2, purity 98%, Supplementary Materials S8), norathyriol (CAS 3542-72-1, C13H8O6, M.W. 260.2, purity 98%, Supplementary Materials S9), 1,3,5,6-tetrahydroxyxanthone (CAS 5084-31-1, C13H8O6, M.W. 260.2, purity 98%, Supplementary Materials S10) and isojacareubin (CAS 50597-93-8, C18H14O6, M.W. 326.3, purity 97%, Supplementary Materials S11) were purchased from BioBioPha Co., Ltd. 1,3,5,8-Tetrahydroxyxanthone (CAS 2980-32-7, C13H8O6, M.W. 260.2, 98%, Supplementary Materials S12), and isomangiferin (CAS 24699-16-9, C19H18O11, M.W. 422.3, 98%, Supplementary Materials S13) were purchased from Chengdu Biopurify Phytochemicals Ltd. 2-Hydroxyxanthone (CAS 1915-98-6, C13H8O3, M.W. 212.2, purity 98%, Supplementary Materials S14) was purchased from BioBioPha Co., Ltd. 7-O-Methylmangiferin (CAS 31002-12-7, C20H20O11, M.W. 436.1, purity 97%, Supplementary Materials S15), neomangiferin (CAS 64809-67-2, C25H28O16, M.W. 584.5, purity 97%, Supplementary Materials S16) and lancerin (CAS 81991-99-3, C19H18O10, M.W. 406.3, purity 98%, Supplementary Materials S17) were purchased from Chengdu Biopurify Phytochemicals Ltd. Other reagents were of analytical grade.
3.2. PTIO•-Trapping Colorimetric Assay
The PTIO•-trapping assay was conducted based on our previously published method [10]. The experimental procedures are briefly described as following: PTIO• radical was dissolved in phosphate pH 4.5 and pH 7.4 buffer to prepare PTIO• solution; xanthone samples were prepared using methanol. Various volumes of xanthone methanolic were brought to phosphate pH 4.5 and pH 7.4 buffer, then were mixed with PTIO• solution. After incubation for 12 h, the product mixture ware measured at 560 nm on a microplate reader (Multiskan FC, Thermo Scientific, Shanghai, China). The PTIO• inhibition percentage was calculated as follows:
| (1) |
where A0 is the absorbance at 560 nm of the control without the sample, and A is the absorbance at 560 nm of the reaction mixture with the sample. The above experiment was repeated using phosphate buffers with different pH values (including pH 4.5 and 7.4).
3.3. UPLC−ESI−Q−TOF−MS/MS Analysis of Xanthone Reaction Product with PTIO•
UPLC−ESI−Q−TOF−MS/MS spectra of the reaction products of PTIO• with the xanthone were obtained according to our previously described method [47]. The methanolic solutions of phenolic components were mixed with a solution of PTIO• radicals in methanol at a molar ratio of 1:2, and the resulting mixtures were incubated for 24 h at room temperature. The product mixtures were filtered through a 0.22-μm filter and measured using a UPLC-ESI-Q-TOF-MS/MS system equipped with a C18 column (2.0 mm i.d. × 100 mm, 2.2 μm, Shimadzu Co., Kyoto, Japan). The mobile phase was used for elution and consisted of a mixture of methanol (phase A) and water (phase B). The column was eluted at a flow rate of 0.3 mL/min with the following gradient elution program: 0–10 min, 60–100% A; 10–15 min, 100% A. The sample injection volume was set at 1 μL to separate the components, and the column temperature was 40 °C. The Q-TOF-MS/MS analysis was conducted on a Triple TOF 5600+ mass spectrometer (AB SCIEX, Framingham, MA, USA) equipped with an ESI source, which was run in the negative ionization mode. The scan range was set at 50–1600 Da. The system was run with the following parameters: ion spray voltage, −4500 V; ion source heater, 550 °C; curtain gas (CUR, N2), 30 psi; nebulizing gas (GS1, air), 50 psi; and TurboIonSpray (TIS) gas (GS2, air), 50 psi. The declustering potential (DP) was set at −100 V, and the collision energy (CE) was set at −40 V with a collision energy spread (CES) of 20 V. The RAF products were quantified by the extracting the corresponding formula (e.g., [C26H14O12-H]− for the norathyriol-norathyriol dimer) from the total ion chromatogram and integrating the corresponding peaks [48,49].
3.4. Statistical Analysis
Each experiment was performed in triplicate and the data were recorded as mean ± SD (standard deviation). The dose–response curves were plotted using Origin 6.0 professional software (OriginLab, Northampton, MA, USA). The IC50 value was defined as the final concentration of 50% radical inhibition (or relative reducing power) [50]. It was calculated by linear regression analysis, and expressed as the mean ± SD (n = 3). The linear regression was analyzed using Origin 6.0. Determination of significant differences between the mean IC50 values was performed using one-way ANOVA and the t-test. The analysis was performed using SPSS software 13.0 for Windows (SPSS Inc., Chicago, IL, USA). p < 0.05 was considered to be statistically significant.
4. Conclusions
Phenolic xanthones may react via ET plus H+-transfer to present antioxidant activity. Through these mechanisms, most xanthones can further dimerize unless they bear huge substituents with steric hindrance. The antioxidant activity of phenolic xanthones is governed by any of four substituent types, i.e., para-di-OHs, 5,6-di-OHs, 6,7-di-OHs, and 7,8-di-OHs. The effects of other types of substituents are very limited. In general, isoprenyl and 3-hydroxy-3-methylbutyl substituents can slightly enhance ET potential. Cyclized-isoprenyl, methyl, and glycoside substituents can cut down phenolic -OH to lower antioxidant activity. However, if these substituents break the aforementioned four types, their detrimental effect may be enhanced.
Acknowledgments
None.
Abbreviations
The following abbreviations are used in this manuscript:
| ABTS•+ | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) |
| DPPH• | 1,1-diphenyl-2-picryl-hydrazl radical |
| ET | electron transfer |
| ET-PT | electron transfer−proton transfer |
| HAT | hydrogen atom transfer |
| PCET | proton coupled electron transfer |
| PTIO• | 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide radical |
| RAF | radical adduct formation |
| ROS | reactive oxygen species |
| SD | standard deviation |
| SPLET | sequential proton-loss electron-transfer |
| Trolox | (±)-6-hydroxy-2,5,7,8-tetramethlychromane-2-carboxylic acid |
| UPLC−ESI−Q−TOF−MS/MS | ultra-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry |
Supplementary Materials
The following are available online. Supplementary Materials S1 Dose response curves and IC50 values; Supplementary Materials S2–17 Analysis certificates and appearances of 16 xanthones. Supplementary Materials S18 Original MS spectra; Supplementary Materials S19 dimerization reaction of norathyriol and MS spectra elucidation; Supplementary Materials S20 dimerization reaction of 1,3,5,6-tetrahydroxyxanthone and MS spectra elucidation.
Author Contributions
X.L. conceived and D.C. designed the experiments; B.C. conducted the experiments; X.L. wrote the paper. All authors read and approved the final manuscript.
Funding
This work was supported by Guangdong Provincial Education Office Science and Technology Project (2017KCXTD007), National Nature Science Foundation of China (81573558), Guangdong Science and Technology Project (2017A050506043), and Natural Science Foundation of Guangdong Province (2017A030312009).
Conflicts of Interest
The authors declare that they have no competing interests.
Footnotes
Sample Availability: Sample of the mangiferin is available from the authors.
References
- 1.Han Q.B., Yang L., Wang Y.L., Qiao C.F., Song J.Z., Sun H.D., Xu H.X. A pair of novel cytotoxic polyprenylated xanthone epimers from gamboges. Chem. Biodivers. 2006;3:101–105. doi: 10.1002/cbdv.200690000. [DOI] [PubMed] [Google Scholar]
- 2.Li H.L., Li X.M., Liu H., Meng L.H., Wang B.G. Two New Diphenylketones and a New Xanthone from Talaromyces islandicus EN-501, an Endophytic Fungus Derived from the Marine Red Alga Laurencia okamurai. Mar. Drugs. 2016;14:223. doi: 10.3390/md14120223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang R., Li P., Li N., Zhang Q., Bai X., Wang L., Xiao Y., Sun L., Yang Q., Yan J. Xanthones from the Pericarp of Garcinia mangostana. Molecules. 2017;22:683. doi: 10.3390/molecules22050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung H.A., Su B.N., Keller W.J., Mehta R.G., Kinghorn A.D. Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen) J. Agric. Food Chem. 2006;54:2077–2082. doi: 10.1021/jf052649z. [DOI] [PubMed] [Google Scholar]
- 5.Ramirez J.E., Zambrano R., Sepúlveda B., Simirgiotis M.J. Antioxidant Properties and Hyphenated HPLC-PDA-MS Profiling of Chilean Pica Mango Fruits (Mangifera indica L. Cv. piqueño) Molecules. 2014;19:438–458. doi: 10.3390/molecules19010438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schieber A., Berardini N., Carle R. Identification of flavonol and xanthone glycosides from mango (Mangifera indica L. Cv. Tommy Atkins) peels by high-performance liquid chromatography-electrospray ionization mass spectrometry. J. Agric. Food Chem. 2003;51:5006–5011. doi: 10.1021/jf030218f. [DOI] [PubMed] [Google Scholar]
- 7.Panda S.S., Chand M., Sakhuja R., Jain S.C. Xanthones as Potential Antioxidants. Cur. Med. Chem. 2013;20:4481–4507. doi: 10.2174/09298673113209990144. [DOI] [PubMed] [Google Scholar]
- 8.Priya V., Jainu M., Mohan S. Biochemical Evidence for the Antitumor Potential of Garcinia mangostana Linn. On Diethylnitrosamine-Induced Hepatic Carcinoma. Pharmacogn. Mag. 2018;14:186–190. doi: 10.4103/pm.pm_213_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kryston T.B., Georgiev A.B., Pissis P., Georgakilas A.G. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res. 2011;711:193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Li X. 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-Oxide (PTIO•) Radical Scavenging: A New and Simple Antioxidant Assay In Vitro. J. Agric. Food Chem. 2017;65:6288–6297. doi: 10.1021/acs.jafc.7b02247. [DOI] [PubMed] [Google Scholar]
- 11.Amorati R., Valgimigli L. Methods to Measure the Antioxidant Activity of Phytochemicals and Plant Extracts. J. Agric. Food Chem. 2018;66:3324–3329. doi: 10.1021/acs.jafc.8b01079. [DOI] [PubMed] [Google Scholar]
- 12.Litwinienko G., Ingold K.U. Solvent Effects on the Rates and Mechanisms of Reaction of Phenols with Free Radicals. Acc. Chem. Res. 2007;40:222–230. doi: 10.1021/ar0682029. [DOI] [PubMed] [Google Scholar]
- 13.Qin H.L., Yu D.Q. 1H-NMR Spectroscopic Databook of Natural Products. 1st ed. Chemical Industry Press; Beijing, China: 2011. pp. 881–2235. [Google Scholar]
- 14.Yang J.S. 13C-NMR Spectroscopic Databook of Natural Products. Chemical Industry Press; Beijing, China: 2011. pp. 1630–2285. [Google Scholar]
- 15.Gómez-Zaleta B., Ramírez-Silva M.T., Gutiérrez A., González-Vergara E., Güizado-Rodríguez M., Rojas-Hernández A. UV/vis, 1H, and 13C NMR spectroscopic studies to determine mangiferin pKa values. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006;64:1002–1009. doi: 10.1016/j.saa.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Bondet V., Brand-Williams W., Berset C. Kinetics and mechanisms of antioxidant activity using the DPPH free radical method. LWT-Food Sci. Technol. 1997;30:609–615. doi: 10.1006/fstl.1997.0240. [DOI] [Google Scholar]
- 17.Krishnamachari V., Levine L.H., Zhou C., Pare P.W. In vitro flavon-3-ol oxidation mediated by a B ring hydroxylation pattern. Chem. Res. Toxicol. 2004;17:795–804. doi: 10.1021/tx034242z. [DOI] [PubMed] [Google Scholar]
- 18.Fourre I., Di Meo F., Podloucka P., Otyepka M., Trouillas P. Dimerization of quercetin, Diels-Alder vs. radical-coupling approach: A joint thermodynamics, kinetics, and topological study. J. Mol. Model. 2016;22:190. doi: 10.1007/s00894-016-3051-8. [DOI] [PubMed] [Google Scholar]
- 19.Krishnamachari V., Levine L.H., Pare P.W. Flavonoid oxidation by the radical generator AIBN: A unified mechanism for quercetin radical scavenging. J. Agric. Food Chem. 2002;50:4357–4363. doi: 10.1021/jf020045e. [DOI] [PubMed] [Google Scholar]
- 20.Burton G.W., Doba T., Gabe E., Ingold K.U. Autoxidation of biological molecules. 4. Maximizing the antioxidant activity of phenols. J. Am. Chem. Soc. 1985;107:7053–7065. doi: 10.1021/ja00310a049. [DOI] [Google Scholar]
- 21.Lucarini M., Pedulli G.F. Free radical intermediates in the inhibition of the autoxidation reaction. Chem. Soc. Rev. 2010;39:2106–2119. doi: 10.1039/b901838g. [DOI] [PubMed] [Google Scholar]
- 22.Heim K.E., Tagliaferro A.R., Bobilya D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002;13:572–584. doi: 10.1016/S0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 23.Li X., Li K., Xie H., Xie Y., Li Y., Zhao X., Jiang X., Chen D. Antioxidant and Cytoprotective Effects of the Di-O-Caffeoylquinic Acid Family: The Mechanism, Structure-Activity Relationship, and Conformational Effect. Molecules. 2018;23:222. doi: 10.3390/molecules23010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De La Cruz J.P., Ruiz-Moreno M.I., Guerrero A., Lopez-Villodres J.A., Reyes J.J., Espartero J.L., Labajos M.T., Gonzalez-Correa J.A. Role of the catechol group in the antioxidant and neuroprotective effects of virgin olive oil components in rat brain. J. Nutr. Biochem. 2015;26:549–555. doi: 10.1016/j.jnutbio.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Li X., Wu X., Huang L. Correlation between antioxidant activities and phenolic contents of radix angelicae sinensis (danggui) Molecules. 2009;14:5349–5361. doi: 10.3390/molecules14125349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J., Li X., Lin J., Li Y., Wang T., Jiang Q., Chen D. Sarcandra glabra (Caoshanhu) protects mesenchymal stem cells from oxidative stress: A bioevaluation and mechanistic chemistry. BMC Complement. Altern. Med. 2016;16:423. doi: 10.1186/s12906-016-1383-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X., Xie Y., Xie H., Yang J., Chen D. π-π Conjugation Enhances Oligostilbene’s Antioxidant Capacity: Evidence from α-Viniferin and Caraphenol A. Molecules. 2018;23:694. doi: 10.3390/molecules23030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali H.M., Ali I.H. Energetic and electronic computation of the two-hydrogen atom donation process in catecholic and non-catecholic anthocyanidins. Food Chem. 2018;243:145–150. doi: 10.1016/j.foodchem.2017.09.120. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi L.F., Lago J.H., Tanizaki T.M., Mascio P.D., Kato M.J. Antioxidant activity of prenylated hydroquinone and benzoic acid derivatives from Piper crassinervium Kunth. Phytochemistry. 2006;67:1838–1843. doi: 10.1016/j.phytochem.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Dong L.M., Jia X.C., Luo Q.W., Zhang Q., Luo B., Liu W.B., Zhang X., Xu Q.L., Tan J.W. Phenolics from Mikania micrantha and Their Antioxidant Activity. Molecules. 2017;22:1140. doi: 10.3390/molecules22071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodman O., Meeker W., Boujaoude M. Vasorelaxant and antioxidant activity of flavonols and flavones: Structure-activity relationships. J. Cardiovasc. Pharm. 2005;46:302–309. doi: 10.1097/01.fjc.0000175431.62626.07. [DOI] [PubMed] [Google Scholar]
- 32.Valgimigli L., Amorati R., Fumo M.G., Dilabio G.A., Pedulli G.F., Ingold K.U., Pratt D.A. The unusual reaction of semiquinone radicals with molecular oxygen. J. Org. Chem. 2008;73:1830–1841. doi: 10.1021/jo7024543. [DOI] [PubMed] [Google Scholar]
- 33.Li X., Wang L., Han W., Mai W., Han L., Chen D. Amentoflavone protects against hydroxyl radical-induced DNA damage via antioxidant mechanism. Turk. J. Biochem. 2014;39:30–36. doi: 10.5505/tjb.2014.65882. [DOI] [Google Scholar]
- 34.Wang T.T., Li X.C., Li Y.R., Chen D.F. Mechanistic chemistry of extraordinary capacity of salvianolic acid B on oxidatively damaged mesenchymal stem cells. J. Chin. Chem. Soc. 2016;63:924–929. doi: 10.1002/jccs.201600112. [DOI] [Google Scholar]
- 35.Apak R., Ozyurek M., Guclu K., Capanoglu E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016;64:997–1027. doi: 10.1021/acs.jafc.5b04739. [DOI] [PubMed] [Google Scholar]
- 36.Li T., Zhang H. Identification and comparative determination of rhodionin in traditional tibetan medicinal plants of fourteen Rhodiola species by high-performance liquid chromatography-photodiode array detection and electrospray ionization-mass spectrometry. Chem. Pharm. Bull. (Tokyo) 2008;56:807–814. doi: 10.1248/cpb.56.807. [DOI] [PubMed] [Google Scholar]
- 37.Guerreiro E., Kavka J., Giordano O.S. 5,8-Dihydroxy-3,6,7-trimethoxyflavone from Gnaphalium gaudichaudianum. Phytochemistry. 1982;21:2601–2603. doi: 10.1016/0031-9422(82)85269-2. [DOI] [Google Scholar]
- 38.Briante R., Ferdinando Febbraio A., Nucci R. Antioxidant Properties of Low Molecular Weight Phenols Present in the Mediterranean Diet. J. Agric. Food Chem. 2003;51:6975–6981. doi: 10.1021/jf034471r. [DOI] [PubMed] [Google Scholar]
- 39.Markovic S., Tosovic J. Comparative study of the antioxidative activities of caffeoylquinic and caffeic acids. Food Chem. 2016;210:585–592. doi: 10.1016/j.foodchem.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H., Wu W., Mo Y. Study of proton-coupled electron transfer (PCET) with four explicit diabatic states at the ab initio level. Comput. Theor. Chem. 2017;1116:50–58. doi: 10.1016/j.comptc.2017.02.005. [DOI] [Google Scholar]
- 41.Amorati R., Baschieri A., Morroni G., Gambino R., Valgimigli L. Peroxyl Radical Reactions in Water Solution: A Gym for Proton-Coupled Electron-Transfer Theories. Chem. Eur. J. 2016;22:7924–7934. doi: 10.1002/chem.201504492. [DOI] [PubMed] [Google Scholar]
- 42.Chen D., Li X., Xu Z., Liu X., Du S., Li H., Zhou J., Zeng H., Hua Z. Hexadecanoic Acid from Buzhong Yiqi Decoction Induced Proliferation of Bone Marrow Mesenchymal Stem Cells. J. Med. Food. 2010;13:967–975. doi: 10.1089/jmf.2009.1293. [DOI] [PubMed] [Google Scholar]
- 43.Li X., Hu Q., Jiang S., Li F., Lin J., Han L., Hong Y., Lu W., Gao Y., Chen D. Flos Chrysanthemi Indici protects against hydroxyl-induced damages to DNA and MSCs via antioxidant mechanism. J. Saudi. Chem. Soc. 2015;19:454–460. doi: 10.1016/j.jscs.2014.06.004. [DOI] [Google Scholar]
- 44.Lin J., Li X., Chen L., Lu W., Chen X., Han L., Chen D. Protective effect against hydroxyl radical-induced DNA damage and antioxidant mechanism of [6]-gingerol: A. Chemical Study. Bull. Korean Chem. Soc. 2014;35:1633–1638. doi: 10.5012/bkcs.2014.35.6.1633. [DOI] [Google Scholar]
- 45.Li X., Han L., Li Y., Zhang J., Chen J., Lu W., Zhao X., Lai Y., Chen D., Wei G. Protective Effect of Sinapine against Hydroxyl Radical-Induced Damage to Mesenchymal Stem Cells and Possible Mechanisms. Chem. Pharm. Bull. (Tokyo) 2016;64:319–325. doi: 10.1248/cpb.c15-00850. [DOI] [PubMed] [Google Scholar]
- 46.Li X., Liu J., Zhao Z., Wang T., Lin J., Chen D. Effects of Natural Chalcone-Tannin Hybrids Protecting Mesenchymal Stem Cells against ROS-mediated Oxidative Damage and Indexes for Antioxidant Mechanisms. Chem. Lett. 2016;45:743–745. doi: 10.1246/cl.160177. [DOI] [Google Scholar]
- 47.Marin M., Manez S. Recent trends in the pharmacological activity of isoprenyl phenolics. Curr. Med. Chem. 2013;20:272–279. doi: 10.2174/092986713804806676. [DOI] [PubMed] [Google Scholar]
- 48.Xie H., Li X., Ren Z., Qiu W., Chen J., Jiang Q., Chen B., Chen D. Antioxidant and cytoprotective effects of Tibetan tea and its phenolic components. Molecules. 2018;23:179. doi: 10.3390/molecules23020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X., Xie Y., Li K., Wu A., Xie H., Guo Q., Xue P., Maleshibek Y., Zhao W., Guo J., et al. Antioxidation and Cytoprotection of Acteoside and Its Derivatives: Comparison and Mechanistic Chemistry. Molecules. 2018;23:498. doi: 10.3390/molecules23020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang Q., Li X.C., Tian Y.G., Lin Q.Q., Xie H., Lu W.B., Chi Y.G., Chen D.F. Lyophilized Aqueous Extracts of Mori Fructus and Mori Ramulus Protect Mesenchymal Stem Cells from •OH-Treated Damage: Bioassay and Antioxidant Mechanism. BMC Complement. Altern. Med. 2017;17:242. doi: 10.1186/s12906-017-1730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.