Abstract
The waste of Sterculia nobilis fruit was massively produced during food processing, which contains lots of natural antioxidants. In this study, antioxidants in the Sterculia nobilis fruit waste were extracted using the green microwave-assisted extraction (MAE) technique. The effects of five independent variables (ethanol concentration, solvent/material ratio, extraction time, temperature, and microwave power) on extraction efficiency were explored, and three major factors (ethanol concentration, extraction time, and temperature) showing great influences were chosen to study their interactions by response surface methodology. The optimal conditions were as follows: 40.96% ethanol concentration, 30 mL/g solvent/material ratio, 37.37 min extraction time at 66.76 °C, and 700 W microwave power. The Trolox equivalent antioxidant capacity value obtained in optimal conditions was in agreement with the predicted value. Besides, MAE improved the extraction efficiency compared with maceration and Soxhlet extraction methods. Additionally, the phenolic profile in the extract was analyzed by UPLC-MS/MS, and eight kinds of phenolic compounds were identified and quantified, including epicatechin, protocatechuic acid, ferulic acid, gallic acid, p-coumaric acid, caffeic acid, quercetin, and p-hydroxycinnamic acid. This study could contribute to the value-added utilization of the waste from Sterculia nobilis fruit, and the extract could be developed as food additive or functional food.
Keywords: Sterculia nobilis, fruit, waste, antioxidant, phenolic compounds, microwave-assisted extraction
1. Introduction
The imbalance between the production of reactive oxygen species and the antioxidant defense system could induce oxidative stress [1,2]. Oxidative stress could cause cell death and tissue damage, and was related to some diseases, including cardiovascular diseases, diabetes, neuronal disorders, and cancer [3]. There is growing scientific evidence that antioxidants could reduce or prevent the negative effects of oxidative stress on living tissues and inhibit aging processes and the development of many diseases [4,5,6]. In addition, antioxidants also play a vital role in the food industry, since they are required for the preservation of biological materials. Synthetic antioxidants such as BHA and BHT have been found very effective in the preservation of foods and in prolonging the shelf life of foods, but are considered to be controversial by consumers. Recently, natural antioxidants have gained increasing attention due to their low toxicity, and they are widely produced by plants including medicinal plants, fruits, and vegetables [7,8,9,10,11,12,13,14,15]. Hence, extraction and identification of natural antioxidants are very necessary and valuable.
Sterculia nobilis belongs to the family of Sterculiaceae, and is a tropical woody plant [16]. Sterculia nobilis is a valuable medicine for treatment of gastroenteric disorder and bloody flux [17,18]. Sterculia nobilis fruit was rich in protein, fiber, vitamins, amino acids, and trace elements. In addition, it contained lots of polyphenols with strong antioxidant activities. The waste of Sterculia nobilis fruit was massively produced during food processing, and contains lots of natural antioxidants. Thus, to make good use of waste materials, natural antioxidants in the hull of Sterculia nobilis fruit were extracted and identified in this study.
There are several methods that could extract antioxidants from natural materials. Conventional methods included Soxhlet extraction, maceration, and steam distillation, while non-conventional methods included MAE, ultrasound-assisted extraction, subcritical water extraction, and supercritical fluid extraction [19,20,21,22]. Non-conventional methods usually consumed less time and organic solvents than conventional ones. Recently, environmentally friendly and high efficient extraction techniques have been popular. MAE, as a green and efficient extraction method, has gained increasing attention. There are some studies indicating that MAE could reduce extraction time, lower extraction temperature, and achieve a high extraction yield [23,24,25]. The physical principle of the MAE method might be the interaction of materials and microwave energy, as well as the dielectric properties of materials [26,27]. Besides, MAE is maneuverable and inexpensive, and has been selected to extract several bioactive constituents from natural materials [28,29]. Therefore, the MAE method was tested in this study for extracting natural antioxidants from the hull of Sterculia nobilis.
Some independent parameters (ethanol concentration, solvent/material ratio, extraction time, temperature, and microwave power) could influence the efficiency of MAE, respectively, and could also have interaction effects [30,31,32,33,34,35,36]. Recently, the response surface methodology (RSM) was utilized frequently for optimizing extraction conditions, because RSM as a mathematical and statistical tool was efficient and reliable [37,38,39]. In this study, the influences of five variables (ethanol concentration, solvent/material ratio, extraction time, temperature, and microwave power) on extraction efficiency were studied respectively via single-factor experiments. Then, RSM was conducted to investigate the interaction of three influential factors. Additionally, MAE was compared with two conventional extraction methods (maceration and Soxhlet extraction). Furthermore, the natural antioxidants in the extract were identified and quantified by UPLC-MS/MS.
2. Results and Discussion
2.1. Single-Factor Experiment Analysis
2.1.1. Influence of Ethanol Concentration
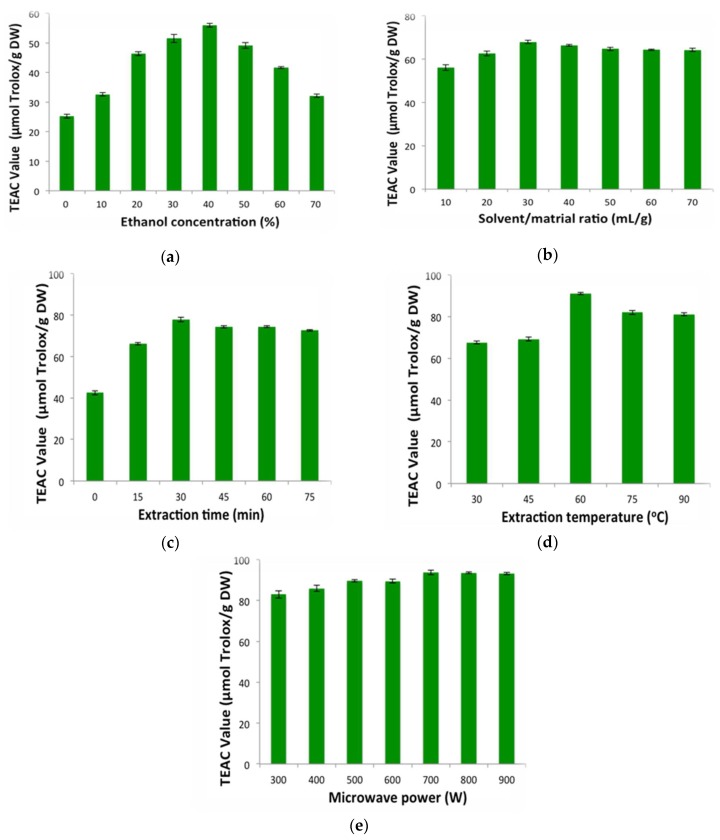
Compared to other organic solvents, aqueous ethanol is safer, cheaper, more easily accessible, and more highly affinitive [40]. Thus, aqueous ethanol was selected as a solvent in this experiment, and toxic methanol and acetone have not been tested in this study. The effect of ethanol concentration on extraction efficiency was studied with solvent/material ratio, 30 mL/g; extraction time, 30 min; temperature, 30 °C; and microwave power, 500 W. It was observed in Figure 1a that Trolox equivalent antioxidant capacity (TEAC) values elevated markedly from 25.16 ± 0.65 to 55.98 ± 1.38 μmol Trolox/g DW, in which ethanol concentration ranged from 0% to 40%. The peak of TEAC value was discovered when ethanol concentration was 40%. Following this, a significant decrease was found with higher ethanol concentration from 50% to 70%. Therefore, 40% ethanol was used in the following experiments.
Figure 1.
The influence of ethanol concentration (a), solvent/material ratio (b), extraction time (c), temperature (d), and microwave power (e) on extraction efficiency.
2.1.2. Influence of Solvent/Material Ratio
Solvent/material ratio (10:1, 20:1, 30:1, 40:1, 50:1, 60:1, and 70:1, mL/g) was chosen to study its impact on the TEAC values of the extract from the hull of Sterculia nobilis fruit. Other four variables were designed as follows: 40% ethanol concentration, 30 min at 30 °C, and 500 W microwave power. The data are displayed in Figure 1b. The TEAC values improved gradually when the solvent/material ratio ranged from 10:1 to 30:1. Then, it was almost unchanged with the further solvent/material ratio enhancement from 30:1 to 70:1. Theoretically, a higher solvent/material ratio could provoke bigger concentration difference and promote mass transfer and diffusion [35]. Nevertheless, elevation of solvent/material ratio might have had little influence on extraction efficiency when the mass transfer reached its peak. Similar results were found in the article about extraction of polysaccharides from the Sargassum thunbergii [41]. Thus, 30:1 was the optimal solvent/material ratio.
2.1.3. Influence of Extraction Time
The impact of extraction time (0, 15, 30, 45, 60, and 75 min) on the TEAC values of the extract was explored with the following design: 40% ethanol concentration, 30 mL/g solvent/material ratio, and 500 W microwave power at 30 °C. It was found in Figure 1c that the TEAC values promoted markedly from 42.47 ± 0.95 to 77.98 ± 1.19 μmol Trolox/g DW with the extraction time prolonged from 0 to 30 min. However, extraction efficiency increased no more and even decreased a little when the duration of microwave was longer. Obviously, microwave could improve the extraction efficiency in a short duration. However, as the exposure time was longer, microwave caused no promoting effects on extraction efficiency or even might have destroyed the antioxidants. Thus, it is suitable to choose 30 min in the following parts.
2.1.4. Influence of Temperature
Figure 1d displayed the impact of temperature (30, 45, 60, 75, and 90 °C) on TEAC values of the sample, which was studied with 40% ethanol concentration, 30 mL/g solvent/material ratio, 30 min extraction time, and 500 W microwave power. It could be observed in Figure 1d that TEAC values increased significantly from 67.63 ± 0.73 to 91.16 ± 0.57 μmol Trolox/g DW with an elevation of the temperature (30 to 60 °C). This phenomenon could be attributed to the diffusion accelerated by suitable high temperature. Following this, a falling trend of extraction efficiency appeared. The reason for this trend might be thermolabile antioxidants decomposed by high temperature (>60°C) [40]. Consequently, 60 °C was the optimal temperature.
2.1.5. Influence of Microwave Power
Microwave power was another important factor affecting extraction efficiency. Different microwave power (300, 400, 500, 600, 700, 800, and 900 W) was studied with four variables designed as 40% ethanol concentration, 30 mL/g solvent/material ratio, and 30 min at 60 °C. According to Figure 1e, the yield of antioxidants presented an uptrend, as the microwave power increased (300 W to 700 W). The TEAC values achieved the maximum (92.67 ± 0.46 μmol Trolox/g DW), while the microwave was 700 W. When microwave power exceeded 700 W, the antioxidant activity slightly decreased. This fact indicated that extraction efficiency could be improved by microwave exposure via accelerating the movement of solvents, diffusion of antioxidants, and breaking of cell wall. Besides, microwave had negative effects of inducing degradation of antioxidants [42,43]. When microwave power was not extremely high, beneficial effects of microwave were greater than negative effects, and extraction efficiency could be elevated. Hence, 700 W was the suitable microwave power.
2.2. RSM Analysis
2.2.1. Central Composite Rotatable Design (CCRD) and Results
RSM using CCRD design was chosen for further optimization of the extraction efficiency of antioxidants from the hull of Sterculia nobilis. Three parameters (ethanol concentration, extraction time, and temperature) that made greater influences on extraction efficiency than other factors were selected. The middle level of these factors was 40% ethanol concentration, 30 min extraction time, and 60 °C temperature, which were obtained from the single-factor experiments. Besides, the other two factors were controlled as 30 mL/g solvent/material ratio and 700 W microwave power. The data were displayed in the Table 1, which included 20 runs experiment designs, actual values, and corresponding predicted values. The data showed that the actual TEAC values varied from 46.08 to 94.25 μmol Trolox/g DW.
Table 1.
The experimental design and results of RSM.
| Run | X1 (Ethanol Concentration, %) | X2 (Extraction Time, min) | X3 (Temperature, °C) | Y (TEAC Value, μmol Trolox/g DW) | |
|---|---|---|---|---|---|
| Actual Value | Predicted Value | ||||
| 1 | 40 | 55.23 | 60 | 80.50 | 82.38 |
| 2 | 40 | 30 | 60 | 92.64 | 89.16 |
| 3 | 30 | 45 | 70 | 88.26 | 85.23 |
| 4 | 30 | 15 | 50 | 46.08 | 46.38 |
| 5 | 30 | 15 | 70 | 76.50 | 71.37 |
| 6 | 40 | 30 | 76.82 | 80.73 | 86.10 |
| 7 | 40 | 30 | 60 | 94.25 | 89.16 |
| 8 | 23.18 | 40 | 60 | 65.21 | 70.08 |
| 9 | 30 | 45 | 50 | 67.75 | 65.55 |
| 10 | 40 | 30 | 60 | 93.10 | 89.16 |
| 11 | 50 | 45 | 70 | 89.64 | 87.01 |
| 12 | 40 | 30 | 60 | 90.79 | 89.16 |
| 13 | 40 | 4.77 | 60 | 54.38 | 55.78 |
| 14 | 50 | 45 | 50 | 65.44 | 68.25 |
| 15 | 40 | 30 | 60 | 92.41 | 89.16 |
| 16 | 50 | 15 | 70 | 74.66 | 74.54 |
| 17 | 40 | 30 | 43.18 | 51.39 | 49.31 |
| 18 | 40 | 30 | 60 | 72.36 | 89.16 |
| 19 | 56.82 | 30 | 60 | 76.60 | 75.02 |
| 20 | 50 | 15 | 50 | 49.77 | 50.48 |
2.2.2. Fitting the Model
A quadratic regression equation was obtained by analysis of the data in Table 1. Equation (1) showed the combined influences of three parameters (ethanol concentration (X1), extraction time (X2), and temperature (X3)) on TEAC values (Y).
| Y = −417.92 + 5.05X1 + 3.04X2 + 10.56X3 − 0.0023X1X2 − 0.0023X1X3 − 0.0088X2X3 − 0.059X12 − 0.032X22 − 0.076X32 | (1) |
Analysis of variance (ANOVA) was used to examine the reliability of the fitting model. As shown in Table 2, the high F value (10.06) and very low p value of 0.0006 (<0.001) of the model indicated that this regression model was credible. Besides, low F value of 0.35 and high p value of 0.8657 suggested that “lack of fit” was not significant, which further confirmed that the model was valid and able to predict the variation precisely. Additionally, according to determination coefficient (R2) (0.9006) and the adjusted R2 (0.8110), the regression model could elucidate almost 81.10% of the response value variations. For the three parameters, both the linear and quadratic effects of extraction time (X2) and temperature (X3) were significant (p < 0.05) on the TEAC values, while the ethanol concentration (X1) only presented a significant (p < 0.05) quadratic effect.
Table 2.
ANOVA for the response surface model.
| Source | Sum of Squares | df | Mean Square | F Value | p Value | Significant |
|---|---|---|---|---|---|---|
| Model | 4252.35 | 9 | 472.48 | 10.06 | 0.0006 | significant |
| X1 | 29.51 | 1 | 29.51 | 0.63 | 0.4463 | |
| X2 | 854.21 | 1 | 854.21 | 18.19 | 0.0016 | |
| X3 | 1633.58 | 1 | 1633.58 | 34.79 | 0.0002 | |
| X1X2 | 0.97 | 1 | 0.97 | 0.021 | 0.8888 | |
| X1X3 | 0.42 | 1 | 0.42 | 0.009 | 0.9262 | |
| X2X3 | 14.04 | 1 | 14.04 | 0.30 | 0.5964 | |
| X12 | 497.42 | 1 | 497.42 | 10.59 | 0.0087 | |
| X22 | 726.50 | 1 | 726.50 | 15.47 | 0.0028 | |
| X32 | 829.77 | 1 | 829.77 | 17.67 | 0.0018 | |
| Residual | 469.59 | 10 | 46.96 | |||
| Lack of Fit | 120.65 | 5 | 24.13 | 0.35 | 0.8657 | not significant |
| Pure Error | 348.94 | 5 | 69.79 | |||
| Cor Total | 4721.94 | 19 | ||||
| R-Squared | 0.9006 | |||||
| Adj R-Squared | 0.8110 |
2.2.3. Response Surfaces Analysis
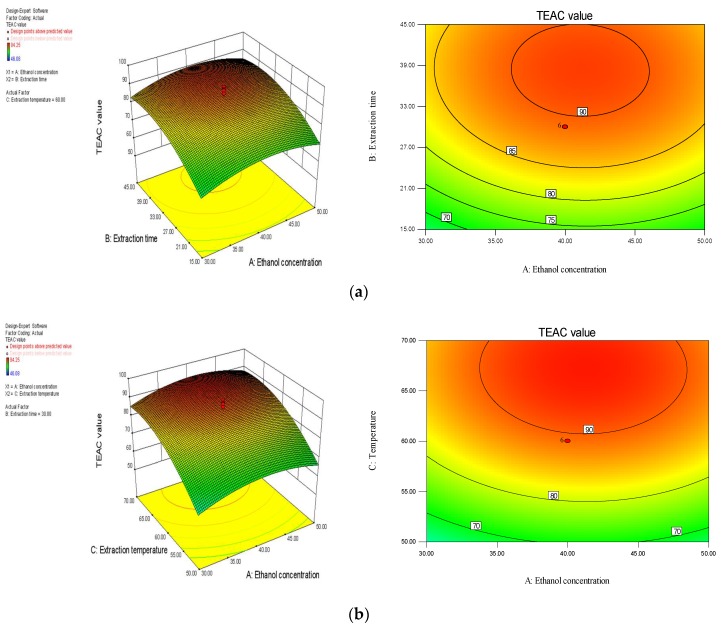
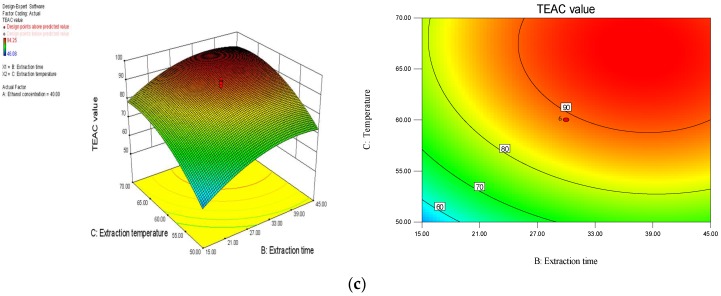
The interaction between the response value (Y, TEAC value) and major parameters (X1, ethanol concentration; X2, extraction time; X3, temperature) can be seen directly from the three-dimensional response surface plots and contour plots. As displayed in Figure 2a, the response value improved with the longer extraction time, when the temperature was 60 °C. However, as the extraction time exceeded 38 min, a decrease of response value was found. Besides, the increasing ethanol concentration (from 30% to 40%) induced an increase of response value. It was observed that the extraction time had a greater influence on the TEAC value than ethanol concentration. In Figure 2b, when the extraction time was set as 30 min, initial increase of extraction temperature (from 50 °C to 67 °C) exerted a great elevation of response value. Then, the response value decreased with the further elevation of temperature. The impact of ethanol concentration on the response value in Figure 2b was similar to that seen in Figure 2a. In Figure 2c, ethanol concentration was fixed at 40%, and the interaction between the extraction time and temperature on the TEAC value was elucidated. The effects of the extraction time and temperature on the TEAC value were similar to those in Figure 2a,b. It was found that the temperature showed a greater impact on the TEAC value than the ethanol concentration and extraction time, according to the analysis of ANOVA and the response surface plots.
Figure 2.
Interaction effects of ethanol concentration (%) and extraction time (min) (a); ethanol concentration and temperature (°C) (b); and extraction time and temperature (c) on TEAC value (μmol Trolox/g DW).
2.2.4. Validation of Predicted Value
The most suitable microwave extraction conditions in the study were as follows: 40.96% ethanol concentration, 30 mL/g solvent/material ratio, 37.37 min extraction time at 66.76 °C, and 700 W microwave power, which were obtained by analyzing the model. The predicted value of the highest TEAC was 94.88 μmol Trolox/g DW according to the model. The validation experiment under the optimal conditions was conducted to examine the reliability of the model. The TEAC actual value of the verification experiment was 93.72 ± 1.05 μmol Trolox/g DW, which corresponded closely to the predicted value. Therefore, it was reliable to use RSM for optimizing extraction efficiency.
2.3. Comparison of MAE with Conventional Methods
In this part, a comparison of MAE with two conventional extraction methods including maceration method and Soxhlet extraction was carried out. As shown in Table 3, in terms of extraction efficiency, the antioxidant activity by MAE (93.72 ± 1.05 μmol Trolox/g DW) was 2.24 times and 3.93 times that acquired by maceration method (41.92 ± 1.96 μmol Trolox/g DW) and Soxhlet extraction (23.84 ± 3.06 μmol Trolox/g DW), respectively. In terms of extraction time, MAE (37.37 min) required significantly less time than maceration method (24 h) and Soxhlet extraction (4 h). Besides, the temperature of Soxhlet extraction (95 °C) was also greatly elevated compared to MAE (66.76 °C). Microwave power polarized the polar molecules and the ionic species, which induced fast volumetric heating via dipolar polarization and ionic conduction and increased reaction rates [27]. This might be the reason for the highest efficiency of MAE among the three approaches. The consequences were similar to two reports related to blueberry leaves and Gordonia axillaris [44,45].
Table 3.
The comparison of MAE with maceration and Soxhlet extraction.
| Extraction Methods | Ethanol Conc. (%) | Extraction Time (min) | Temp. (°C) | TEAC (μmol Trolox/g DW) | TPC (mg GAE/g DW) | TFC (mg QE/g DW) |
|---|---|---|---|---|---|---|
| Maceration | 40.96 | 24 h | 25 | 41.92 ± 1.96 | 2.74 ± 0.69 | 0.30 ± 0.17 |
| Soxhlet | 40.96 | 4 h | 95 | 23.84 ± 3.06 | 2.56 ± 0.64 | 0.24 ± 0.10 |
| MAE | 40.96 | 37.37 | 66.76 | 93.72 ± 1.05 | 3.67 ± 0.80 | 0.45 ± 0.13 |
2.4. Analysis of Phenolic Compounds
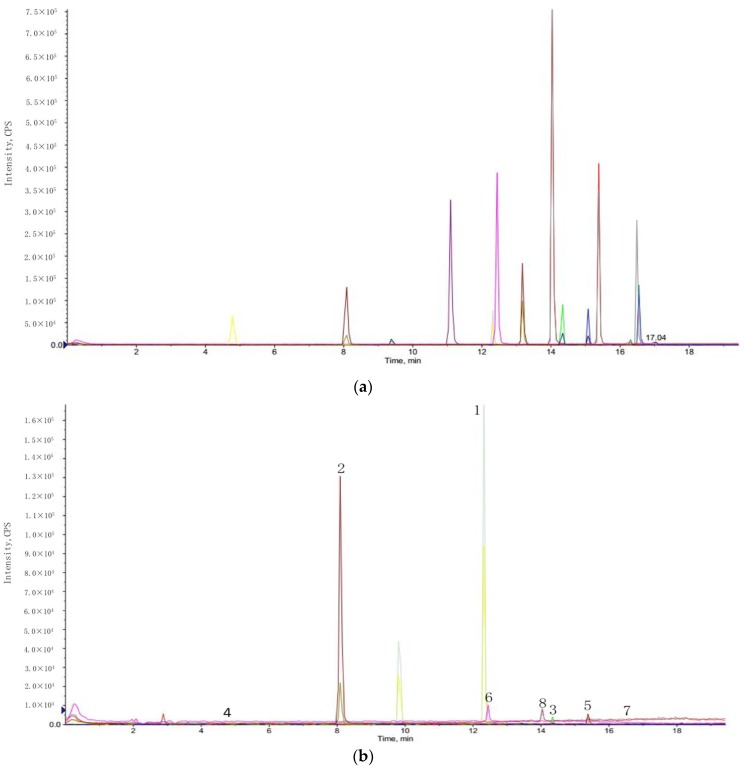
Phenolic compounds showed strong antioxidant activity, and the identification of these components was helpful to understand the antioxidant properties of the extract [46,47]. In this part, the phenolic compounds in the hull of Sterculia nobilis fruit were identified by UPLC-MS/MS. Figure 3 displayed the total ion chromatograms of standard phenolic components and the extract acquired under the optimal conditions. Table 4 showed eight phenolic constituents that existed in the sample. The content of epicatechin was the highest, followed by protocatechuic acid, ferulic acid, gallic acid, p-coumaric acid, and caffeic acid. The antioxidant capacity of the hull of Sterculia nobilis fruit might be attributed to these phenolic components synergistically. In addition, these phenolic components also had other bioactivities, including antibacterial, anti-inflammatory, and anticancer effects [48,49,50]. Hence, the hull of Sterculia nobilis fruit might possess similar bioactivities and potential health benefits.
Figure 3.
The total ion chromatograms of standard phenolic components (a) and the extract acquired under the optimal conditions (b).
Table 4.
The contents of phenolic compounds in the extract acquired under the optimal condition.
| Number | Phenolic Compounds | Retention Time (tR, min) | Paront Ion (m/z, [M − H]−) | Product Ion (m/z) | Contents (μg/g DW) |
|---|---|---|---|---|---|
| 1 | Epicatechin | 12.3 | 289 | 203 | 56.63 ± 0.58 |
| 2 | Protocatechuic acid | 8.08 | 153.1 | 109 | 21.09 ± 0.16 |
| 3 | Ferulic acid | 14.3 | 193.1 | 134 | 0.84 ± 0.003 |
| 4 | Gallic acid | 4.79 | 169.1 | 125 | 0.53 ± 0.008 |
| 5 | p-Coumaric acid | 15.4 | 162.7 | 119 | 0.45 ± 0.003 |
| 6 | Caffeic acid | 12.4 | 179.1 | 135 | 0.35 ± 0.010 |
| 7 | Quercetin | 16.5 | 301 | 179 | 0.041 ± 0.001 |
| 8 | p-Hydroxycinnamic acid | 14 | 163.1 | 119 | 0.027 ± 0.001 |
3. Materials and Methods
3.1. Chemicals and Reagents
Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) and ABTS (2,20-azinobis(3-ethyl-benothiazoline-6-sulphonic acid) diammonium salt) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Potassium persulphate was obtained from Tianjin Chemical Factory (Tianjin, China). Ethanol and methanol were purchased from Kelong Chemical Factory (Chengdu, China). Chemicals and reagents used in the study were analytically pure and deionized water was used.
3.2. Instruments
The MAE was conducted in an X-100A microwave extraction device (Xianghu Instrumental Company, Beijing, China) with a 1000 W microwave power, which had temperature monitor and microprocessor programmer software that could regulate experiment variables including extraction temperature, time, and microwave power.
3.3. Sample Preparation
The hull of Sterculia nobilis fruit was collected and dried at room temperature. Then, the hull was ground into fine particles that were smaller than 0.300 mm by a food grinder (RHP-100, Ronghao Industry & Trade Co. Ltd., Yongkang, China) and was stored at 4 °C in a refrigerator until utilized.
3.4. Extraction of Antioxidants
3.4.1. Microwave-Assisted Extraction
The ground powder of the hulls of Sterculia nobilis fruit (0.05 g) was put in a capped tube. Proper aqueous ethanol was added to the powder. The mixture was shaken for 5 min in vortex mixer to soak the mixture completely. Then, the sample was placed into a water bath of the microwave device. After irradiation with the pre-set parameters (extraction time, temperature, and microwave power), the tube containing the sample was centrifuged at 4200 g for 15 min, and the TEAC value of supernatant was determined.
3.4.2. Maceration Extraction
Firstly, the ground powder of the hulls (0.05 g) was placed in a capped tube. Then, 15 mL of 40.96% ethanol was added to the powder. After 24 h maceration in a shaking water bath at 25 °C, the mixture in the tube was centrifuged at 4200 g for 15 min, and the supernatant was collected for subsequent determination.
3.4.3. Soxhlet Extraction
The Soxhlet extraction referred to the experimental process designed by Xu et al. [51]. The ground powder of the hulls (2.0 g) was wrapped by Whatman filter paper, with 400 mL of 40.96% ethanol in Soxhlet extractor. After 4 h extraction at 95 °C, the extraction solution was obtained for next analysis.
3.5. Determination of Antioxidant Capacity
There are several methods that could evaluate the antioxidant capacity of the sample, but the results gained using different methods generally have a very high correlation [7,12]. The TEAC assay was frequently used for evaluation of antioxidant capacity owing to its simplicity and rapidness [7]. The antioxidant activity of the extract was evaluated by TEAC assay according to previous procedure [52]. Tersely, the ABTS•+ stock solution was prepared by mixing potassium persulfate (2.45 μmol/L) and ABTS•+ (7 μmol/L) with a volume ratio of 1:1. Then, the ABTS•+ stock solution was incubated for 16 h in dark environment at room temperature and was utilized within 48 h. The ABTS•+ stock solution was diluted to insure that the absorbance of ABTS•+ working solution was 0.70 ± 0.05 at 734 nm. Finally, 3.8 mL ABTS•+ working solution was added to 100 μL diluted sample, and the absorbance was determined at 734 nm after incubation 6 min in dark environment at normal temperature. The results were expressed as μmol Trolox/g DW.
3.6. Determination of Total Phenolic Content
The method of determination of total phenolic content (TPC) in the extract was referred to the previous article [13]. The gallic acid was employed for reference standard, and the results were stated as mg gallic acid equivalent (GAE)/g DW.
3.7. Determination of Total Flavonoid Content
The total flavonoid content (TFC) in the extract was measured according to the established process [53]. The quercetin was selected as the reference standard, and the results were stated as mg quercetin equivalent (mg QE)/g DW.
3.8. Identification and Quantification of Phenolic Compounds
The phenolic constituents in the sample acquired under optimal conditions were evaluated referring to the procedure established by Zhou et al. with a little modification [52]. Phenolic compounds were identified and quantified by AB Sciex 4000 Qtrap liquid chromatography-tandem mass spectrometry (SCIEX, Framingham, MA, USA), and Acquity UPLC® HSS T3 column (3.0 × 150 mm, 1.8 μm, Waters, Milford, MA, USA) was used to separate at 40 °C. Solution A (0.2% formic acid aqueous solution) and solution B (methanol) formed the mobile phase, and the flow rate was 0.3 mL/min. The gradient elution was carried out as follows: 0–2 min, 15% (B); 2–8 min, 15–30% (B); 8–15 min, 30–80% (B); 15–17.5 min, 80% (B); and 17.5–19.5 min, 15% (B). The sample was injected with 2 μL. The parameters of mass spectrometry were controlled as follows: ESI source with negative mode, ion source temperature at 550 °C, 4500 V capillary voltage with the mode of multiple reaction monitoring and 10 psig curtain gas, and 20 psig nebulizer gas and 20 psig auxiliary gas. Firstly, the identification of phenolic constituents was conducted by tandem mass spectrometry. Then, verification and quantification of these constituents were acquired by contrasting the retention times and peak areas of corresponding standards.
3.9. Experiment Design
3.9.1. Single-Factor Experiments
Five variables, including ethanol concentration, solvent/material ratio, extraction time, temperature, and microwave power, were selected to evaluate their effects on antioxidant capacity of the extract from the hull of Sterculia nobilis fruit. The levels of each factor were designed as follows: ethanol concentration 20% to 70%, solvent/material ratio 10:1 to 70:1, extraction time 0 to 75 min, temperature 30 to 90 °C, and microwave power 300 to 900 W. Three factors that made significant differences on extraction efficiencies would be chosen to conduct subsequent experiments.
3.9.2. Response Surface Methodology
RSM using CCRD was used to further optimize the antioxidants capacity of the extract from the hull of Sterculia nobilis fruit. As shown in Table 5, three key variables (ethanol concentration (X1), extraction time (X2), and temperature (X3)) were selected and evaluated in 5 levels. 20 experimental runs including 6 replicates in the central point were carried out. The data of CCRD were based on a second-order polynomial model, as follows:
| Y = β0 + ∑βiXi + ∑βiiXi2 + ∑βijXiXj | (2) |
Table 5.
Five variables and their levels of CCRD.
| Variable | Units | Symbol | Code Levels | ||||
|---|---|---|---|---|---|---|---|
| −1.68 | −1 | 0 | 1 | 1.68 | |||
| Ethanol concentration | % (v/v) | X1 | 23.18 | 30 | 40 | 50 | 56.82 |
| Extraction time | min | X2 | 4.77 | 15 | 30 | 45 | 55.23 |
| Temperature | °C | X3 | 43.18 | 50 | 60 | 70 | 76.82 |
3.9.3. Statistical Analysis
All the experiments were conducted in triplicate. The average value ± SD (standard deviation) was reported. Statistical analysis was implemented using Design Expert 8.0.6 and Excel 2016.
4. Conclusions
An environmentally friendly MAE method has been established to extract antioxidants from the hulls of Sterculia nobilis fruit, and RSM was selected to further optimize three experimental factors. The results indicated that the optimal extraction conditions were 40.96% ethanol concentration, 30 mL/g solvent/material ratio, 37.37 min at 66.76 °C, and 700 W microwave power. Under the optimal conditions, the maximum TEAC value obtained was 93.72 ± 1.05 μmol Trolox/g DW, and TPC, as well as TFC of the extract, was 3.67 ± 0.80 and 0.45 ± 0.13 μmol Trolox/g DW, respectively. The high R2 and consistency between the predicted and actual value demonstrated the preciseness and reliability of the model. Additionally, MAE was more efficient at extracting antioxidants from the hulls of Sterculia nobilis fruit compared to two conventional methods. In conclusion, MAE is a promising technique for natural constituents extraction because of its high efficiency and environmentally friendly character. Finally, epicatechin, protocatechuic acid, ferulic acid, gallic acid, p-coumaric acid, caffeic acid, quercetin, and p-hydroxycinnamic acid were identified and quantified, which might contribute greatly to the antioxidant activity of the hulls of Sterculia nobilis fruit. This study could be helpful for the value-added utilization of the waste from Sterculia nobilis fruit, and the extract could be developed as food additive or functional food.
Author Contributions
J.-J.Z. and H.-B.L. conceived and designed the experiments; J.-J.Z., Y.L., and S.-J.L. performed the experiments; J.-J.Z. analyzed the data and wrote the paper; and H.-B.L. revised the paper.
Funding
This research was funded by Key Project of Guangdong Provincial Science and Technology Program grant number [2014B020205002], National Natural Science Foundation of China grant number [81372976] and the Hundred-Talents Scheme of Sun Yat-Sen University.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Grzesik M., Naparlo K., Bartosz G., Sadowska-Bartosz I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018;241:480–492. doi: 10.1016/j.foodchem.2017.08.117. [DOI] [PubMed] [Google Scholar]
- 2.Guo Y.J., Deng G.F., Xu X.R., Wu S., Li S., Xia E.Q., Li F., Chen F., Ling W.H., Li H.B. Antioxidant capacities, phenolic compounds and polysaccharide contents of 49 edible macro-fungi. Food Funct. 2012;3:1195–1205. doi: 10.1039/c2fo30110e. [DOI] [PubMed] [Google Scholar]
- 3.Kandikattu H.K., Rachitha P., Krupashree K., Jayashree G.V., Abhishek V., Khanum F. LC-ESI-MS/MS analysis of total oligomeric flavonoid fraction of Cyperus rotundus and its antioxidant, damage protective and antihemolytic effects. Pathophysiology. 2015;22:165–173. doi: 10.1016/j.pathophys.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Obrenovich M.E., Li Y., Parvathaneni K., Yendluri B.B., Palacios H.H., Leszek J., Aliev G. Antioxidants in health, disease and aging. CNS Neurol. Disord.-Drug Targets. 2011;10:192–207. doi: 10.2174/187152711794480375. [DOI] [PubMed] [Google Scholar]
- 5.Stolarzewicz I.A., Ciekot J., Fabiszewska A.U., Bialecka-Florjanczyk E. Plant and microbial sources of antioxidants. Postep. Hig. Med. Doswiadczalnej. 2013;67:1359–1373. doi: 10.5604/17322693.1083019. [DOI] [PubMed] [Google Scholar]
- 6.Moure A., Cruz J.M., Franco D., Dominguez J.M., Sineiro J., Dominguez H., Nunez M.J., Parajo J.C. Natural antioxidants from residual sources. Food Chem. 2001;72:145–171. doi: 10.1016/S0308-8146(00)00223-5. [DOI] [Google Scholar]
- 7.Fu L., Xu B.T., Xu X.R., Qin X.S., Gan R.Y., Li H.B. Antioxidant capacities and total phenolic contents of 56 wild fruits from South China. Molecules. 2010;15:8602–8617. doi: 10.3390/molecules15128602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song F.L., Gan R.Y., Zhang Y., Xiao Q., Kuang L., Li H.B. Total phenolic contents and antioxidant capacities of selected Chinese medicinal plants. Int. J. Mol. Sci. 2010;11:2362–2372. doi: 10.3390/ijms11062362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu L., Xu B.T., Gan R.Y., Zhang Y.A., Xu X.R., Xia E.Q., Li H.B. Total phenolic contents and antioxidant capacities of herbal and tea infusions. Int. J. Mol. Sci. 2011;12:2112–2124. doi: 10.3390/ijms12042112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu L., Xu B.T., Xu X.R., Gan R.Y., Zhang Y., Xia E.Q., Li H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011;129:345–350. doi: 10.1016/j.foodchem.2011.04.079. [DOI] [PubMed] [Google Scholar]
- 11.Deng G.F., Xu X.R., Zhang Y., Li D., Gan R.Y., Li H.B. Phenolic compounds and bioactivities of pigmented rice. Crit. Rev. Food Sci. 2013;53:296–306. doi: 10.1080/10408398.2010.529624. [DOI] [PubMed] [Google Scholar]
- 12.Deng G.F., Lin X., Xu X.R., Gao L.L., Xie J.F., Li H.B. Antioxidant capacities and total phenolic contents of 56 vegetables. J. Funct. Foods. 2013;5:260–266. doi: 10.1016/j.jff.2012.10.015. [DOI] [Google Scholar]
- 13.Li A.N., Li S., Li H.B., Xu D.P., Xu X.R., Chen F. Total phenolic contents and antioxidant capacities of 51 edible and wild flowers. J. Funct. Foods. 2014;6:319–330. doi: 10.1016/j.jff.2013.10.022. [DOI] [Google Scholar]
- 14.Gan R.Y., Shah N.P., Wang M.F., Lui W.Y., Corke H. Fermentation alters antioxidant capacity and polyphenol distribution in selected edible legumes. Int. J. Food Sci. Technol. 2016;51:875–884. doi: 10.1111/ijfs.13062. [DOI] [Google Scholar]
- 15.Li Y., Zhang J.J., Xu D.P., Zhou T., Zhou Y., Li S., Li H.B. Bioactivities and health benefits of wild fruits. Int. J. Mol. Sci. 2016;17:1258. doi: 10.3390/ijms17081258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y.W., Lu Y.Y., Chen X.L., Ren H., Su W.Q., Liu Y.Q., Luo R.H. Nutritional and health care valuation of seed embryo Sterculia nobilis Smith. J. South. Agric. 2012;43:641–648. [Google Scholar]
- 17.Song J.L., Sun P., Wang R., Zhao X. Gastroprotective effects of methanolic extract of Sterculia nobilis Smith seeds in reserpine-induced gastric ulcer in mice. J. Food Biochem. 2015;39:230–237. doi: 10.1111/jfbc.12125. [DOI] [Google Scholar]
- 18.Ren H., Zhou J., Li Y.W., Wei C.Z., Lu Y.Y., Lu Y.C., Luo R.H. Study on nutrition and antioxidant activities of Sterculia nobilis Smith seeds. Plant Sci. J. 2013;31:203–208. doi: 10.3724/SP.J.1142.2013.20203. [DOI] [Google Scholar]
- 19.Xu D.P., Li Y., Meng X., Zhou T., Zhou Y., Zheng J., Zhang J.J., Li H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017;18:96. doi: 10.3390/ijms18010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y., Zheng J., Gan R., Zhou T., Xu D., Li H. Optimization of ultrasound-assisted extraction of antioxidants from the mung bean coat. Molecules. 2017;22:638. doi: 10.3390/molecules22040638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu D.P., Zheng J., Zhou Y., Li Y., Li S., Li H.B. Ultrasound-assisted extraction of natural antioxidants from the flower of Limonium sinuatum: Optimization and comparison with conventional methods. Food Chem. 2017;217:552–559. doi: 10.1016/j.foodchem.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Li A.N., Li S., Li Y., Xu D.P., Li H.B. Optimization of ultrasound-assisted extraction of natural antioxidants from the Osmanthus fragrans flower. Molecules. 2016;21:218. doi: 10.3390/molecules21020218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H., Ding J., Ren N.Q. Recent advances in microwave-assisted extraction of trace organic pollutants from food and environmental samples. TrAC Trends Anal. Chem. 2016;75:197–208. doi: 10.1016/j.trac.2015.05.005. [DOI] [Google Scholar]
- 24.Sahin S., Samli R., Tan A., Barba F.J., Chemat F., Cravotto G., Lorenzo J.M. Solvent-free microwave-assisted extraction of polyphenols from olive tree leaves: Antioxidant and antimicrobial properties. Molecules. 2017;22:1056. doi: 10.3390/molecules22071056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perino-Issartier S., Abert-Vian M., Chemat F. Solvent free microwave-assisted extraction of antioxidants from sea buckthorn (Hippophae rhamnoides) food by-products. Food Bioprocess Technol. 2011;4:1020–1028. doi: 10.1007/s11947-010-0438-x. [DOI] [Google Scholar]
- 26.Nemes S.M., Orsat V. Screening the experimental domain for the microwave-assisted extraction of secoisolariciresinol diglucoside from flaxseed prior to optimization procedures. Food Bioprocess Technol. 2010;3:300–307. doi: 10.1007/s11947-009-0212-0. [DOI] [Google Scholar]
- 27.Kappe C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004;43:6250–6284. doi: 10.1002/anie.200400655. [DOI] [PubMed] [Google Scholar]
- 28.Xia E.Q., Cui B., Xu X.R., Song Y., Ai X.X., Li H.B. Microwave-assisted extraction of oxymatrine from Sophora flavescens. Molecules. 2011;16:7391–7400. doi: 10.3390/molecules16097391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia E.Q., Wang B.W., Xu X.R., Zhu L., Song Y., Li H.B. Microwave-assisted extraction of oleanolic acid and ursolic acid from Ligustrum lucidum Ait. Int. J. Mol. Sci. 2011;12:5319–5329. doi: 10.3390/ijms12085319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kala H.K., Mehta R., Sen K.K., Tandey R., Mandal V. Critical analysis of research trends and issues in microwave-assisted extraction of phenolics: Have we really done enough. TrAC Trend. Anal. Chem. 2016;85:140–152. doi: 10.1016/j.trac.2016.09.007. [DOI] [Google Scholar]
- 31.Gan C.Y., Latiff A.A. Optimization of the solvent extraction of bioactive compounds from Parkia speciosa pod using response surface methodology. Food Chem. 2011;124:1277–1283. doi: 10.1016/j.foodchem.2010.07.074. [DOI] [Google Scholar]
- 32.Ameer K., Bae S.W., Jo Y., Lee H.G., Ameer A., Kwon J.H. Optimization of microwave-assisted extraction of total extract, stevioside and rebaudioside-A from Stevia rebaudiana (Bertoni) leaves, using response surface methodology (RSM) and artificial neural network (ANN) modelling. Food Chem. 2017;229:198–207. doi: 10.1016/j.foodchem.2017.01.121. [DOI] [PubMed] [Google Scholar]
- 33.Sinha K., Chowdhury S., Das Saha P., Datta S. Modeling of microwave-assisted extraction of natural dye from seeds of Bixa orellana (Annatto) using response surface methodology (RSM) and artificial neural network (ANN) Ind. Crop. Prod. 2013;41:165–171. doi: 10.1016/j.indcrop.2012.04.004. [DOI] [Google Scholar]
- 34.Lefsih K., Giacomazza D., Dahmoune F., Mangione M.R., Bulone D., Biagio P., Passantino R., Costa M.A., Guarrasi V., Madani K., et al. Pectin from Opuntia ficus indica: Optimization of microwave-assisted extraction and preliminary characterization. Food Chem. 2017;221:91–99. doi: 10.1016/j.foodchem.2016.10.073. [DOI] [PubMed] [Google Scholar]
- 35.Yanik D.K. Alternative to traditional olive pomace oil extraction systems: Microwave-assisted solvent extraction of oil from wet olive pomace. LWT-Food Sci. Technol. 2017;77:45–51. doi: 10.1016/j.lwt.2016.11.020. [DOI] [Google Scholar]
- 36.Bhan M., Satija S., Garg C., Dureja H., Garg M. Optimization of ionic liquid-based microwave-assisted extraction of a diterpenoid lactone-andrographolide from Andrographis paniculata by response surface methodology. J. Mol. Liq. 2017;229:161–166. doi: 10.1016/j.molliq.2016.12.011. [DOI] [Google Scholar]
- 37.Saikia S., Mahnot N.K., Mahanta C.L. Optimization of phenolic extraction from Averrhoa carambola pomace by response surface methodology and its microencapsulation by spray and freeze drying. Food Chem. 2015;171:144–152. doi: 10.1016/j.foodchem.2014.08.064. [DOI] [PubMed] [Google Scholar]
- 38.Chen C., Shao Y., Tao Y.D., Wen H.X. Optimization of dynamic microwave-assisted extraction of Armillaria polysaccharides using RSM, and their biological activity. LWT-Food Sci. Technol. 2015;64:1263–1269. doi: 10.1016/j.lwt.2015.07.009. [DOI] [Google Scholar]
- 39.Bezerra M.A., Santelli R.E., Oliveira E.P., Villar L.S., Escaleira L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008;76:965–977. doi: 10.1016/j.talanta.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 40.Dailey A., Vuong Q. Optimization of aqueous extraction conditions for recovery of phenolic content and antioxidant properties from Macadamia (Macadamia tetraphylla) skin waste. Antioxidants. 2015;4:699–718. doi: 10.3390/antiox4040699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren B.B., Chen C., Li C., Fu X., You L.J., Liu R.H. Optimization of microwave-assisted extraction of Sargassum thunbergii polysaccharides and its antioxidant and hypoglycemic activities. Carbohydr. Polym. 2017;173:192–201. doi: 10.1016/j.carbpol.2017.05.094. [DOI] [PubMed] [Google Scholar]
- 42.Ghasemzadeh A., Jaafar H., Rahmat A., Swamy M.K. Optimization of microwave-assisted extraction of zerumbone from Zingiber zerumbet L. rhizome and evaluation of antiproliferative activity of optimized extracts. Chem. Cent. J. 2017;11:5. doi: 10.1186/s13065-016-0235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu B., Li C., Zhang Z.Q., Zhao Q., Zhu Y.D., Su Z., Chen Y.Z. Microwave-assisted extraction of silkworm pupal oil and evaluation of its fatty acid composition, physicochemical properties and antioxidant activities. Food Chem. 2017;231:348–355. doi: 10.1016/j.foodchem.2017.03.152. [DOI] [PubMed] [Google Scholar]
- 44.Routray W., Orsat V. MAE of phenolic compounds from blueberry leaves and comparison with other extraction methods. Ind. Crop. Prod. 2014;58:36–45. doi: 10.1016/j.indcrop.2014.03.038. [DOI] [Google Scholar]
- 45.Li Y., Li S., Lin S.J., Zhang J.J., Zhao C.N., Li H.B. Microwave-assisted extraction of natural antioxidants from the exotic Gordonia axillaris fruit: Optimization and identification of phenolic compounds. Molecules. 2017;22:1481. doi: 10.3390/molecules22091481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiu D.R., Guo J., Yu H.M., Yan J., Yang S.X., Li X., Zhang Y.M., Sun J.Z., Cong J., He S.L., et al. Antioxidant phenolic compounds isolated from wild Pyrus ussuriensis Maxim. fruit peels and leaves. Food Chem. 2017;241:182–187. doi: 10.1016/j.foodchem.2017.08.072. [DOI] [PubMed] [Google Scholar]
- 47.Pang Y.H., Ahmed S., Xu Y.J., Beta T., Zhu Z.W., Shao Y.F., Bao J.S. Bound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice. Food Chem. 2017;240:212–221. doi: 10.1016/j.foodchem.2017.07.095. [DOI] [PubMed] [Google Scholar]
- 48.Mandel S.A., Amit T., Kalfon L., Reznichenko L., Weinreb O., Youdim M.B.H. Cell signaling pathways and iron chelation in the neurorestorative activity of green tea polyphenols: Special reference to epigallocatechin gallate (EGCG) J. Alzheimers Dis. 2008;15:211–222. doi: 10.3233/JAD-2008-15207. [DOI] [PubMed] [Google Scholar]
- 49.Shay J., Elbaz H.A., Lee I., Zielske S.P., Malek M.H., Huttemann M. Molecular mechanisms and therapeutic effects of (−)-epicatechin and other polyphenols in cancer, inflammation, diabetes, and neurodegeneration. Oxid. Med. Cell. Longev. 2015;2015:181260. doi: 10.1155/2015/181260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hosseinzadeh H., Nassiri-Asl M. Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. J. Endocrinol. Investig. 2014;37:783–788. doi: 10.1007/s40618-014-0096-3. [DOI] [PubMed] [Google Scholar]
- 51.Xu D.P., Zheng J., Zhou Y., Li Y., Li S., Li H.B. Extraction of natural antioxidants from the Thelephora ganbajun mushroom by an ultrasound-assisted extraction technique and evaluation of antiproliferative activity of the extract against human cancer cells. Int. J. Mol. Sci. 2016;17:1664. doi: 10.3390/ijms17101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou T., Xu D.P., Lin S.J., Li Y., Zheng J., Zhou Y., Zhang J.J., Li H.B. Ultrasound-assisted extraction and identification of natural antioxidants from the fruit of Melastoma sanguineum Sims. Molecules. 2017;22:306. doi: 10.3390/molecules22020306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalia K., Sharma K., Singh H.P., Singh B. Effects of extraction methods on phenolic contents and antioxidant activity in aerial parts of Potentilla atrosanguinea Lodd. and quantification of its phenolic constituents by RP-HPLC. J. Agric. Food Chem. 2008;56:10129–10134. doi: 10.1021/jf802188b. [DOI] [PubMed] [Google Scholar]