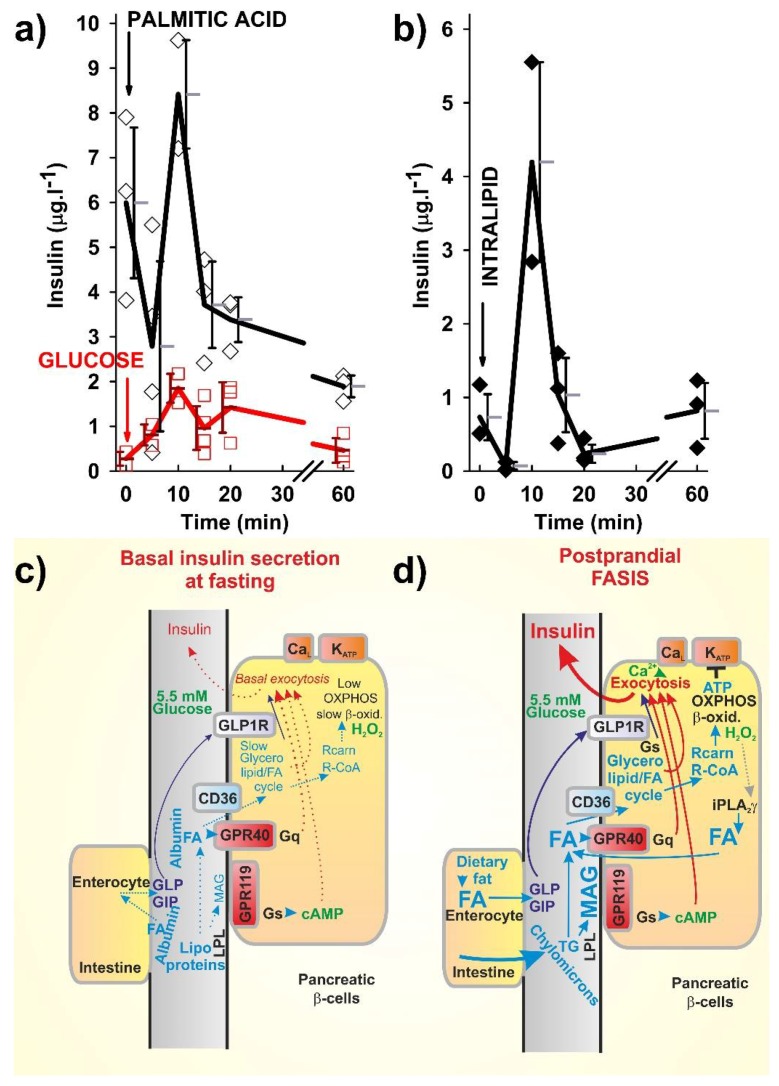
Figure 1.
Fatty acid (FA)-stimulated insulin secretion (FASIS) in mice (a,b) as compared to glucose-stimulated insulin secretion (GSIS), (a) and schemes of incretin-mediated basal insulin secretion at fasting (c) and FASIS at basal glucose levels (d), with a theoretical fat only meal. (a,b) Time course of insulin release into the blood from the eye plexus blood vessel in C57Bl6J mice, fasted 6 h prior to an intraperitoneal (i.p.) injection (arrows) of 0.1 mg palmitic acid (a, black) or 1.5 mg intralipid (b) per 1 g of mice body weight, is plotted as the obtained data or the averages with standard deviations. Alternatively, glucose 1 mg per g body weight was i.p. injected (a, red). Insulin was detected from the blood serum by an insulin kit (Mercodia, Uppsala, Sweden). Approved by the Animal Care and Use Committee (Inst. Molecular Genetics, ASCR), in accordance with the European Union Directive 2010/63/EU. (c,d) Insulin release prior to and after a fatty meal not containing saccharides—predicted mechanisms of negligible fasting fatty acid-stimulated insulin secretion (FASIS) vs. significant postraprandial FASIS: (a) FASIS due to the existence of FAs in plasma during fasting (‘fasting basal FASIS’) is a part of the basal insulin secretion. Such a very low contribution to the insulin released during the fasting state may originate from the FA stimulation of the intenstinal incretin release with subsequent GLP1R- and GIPR-mediated insulin release in β-cells. (b) Postprandial FASIS can also be considered as consisting of the intenstinal and β-cell components—a much higher induction of intenstinal incretin release is now in effect. Solely β-cell-dependent FASIS is now initiated by the lipoprotein lipase cleavage of triglycerides from chylomicrons to long chain C16–C18 FAs (LCFAs) and 2MAG, both stimulating their own receptors (GPR40 and GPR119, respectively), which further stimulates insulin granule exocytosis in KATP-independent ways, as well as evoking metabolic stimulation of the insulin release, after the FA transport into β-cells by the CD36 transporter. The metabolic stimulation proceeds either via the glycerol/FA cycle (part of which stimulates the insulin release in a KATP-independent way via the exocytosis-promoting protein Munc13-1, and part via the corresponding fractional increase in ATP) or after β-oxidation and, subsequently, increased the OXPHOS (hence, ATP) by the KATP-dependent way. Dotted arrows represent low or non-existing signaling or fluxes.