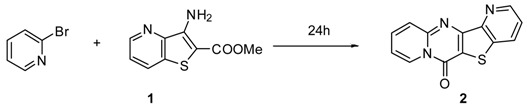
Table 1.
Optimization of conditions to generate tetracyclic compound 2.
| Entry | [Cat] (mol %) | Solvent | Ligand (mol %) | T (°C) | Base 2.62 equiv | Yield (%) [a] |
|---|---|---|---|---|---|---|
| 1 | Pd(OAc)2 (3) | Dioxane | Xantphos (4) | 120 | Cs2CO3 | 67 |
| 2 | Pd(OAc)2 (3) | Dioxane | PPh3 (6) | 120 | Cs2CO3 | 33 |
| 3 | Pd(OAc)2 (5) | Dioxane | 10-phenanthroline (10) | 120 | Cs2CO3 | 18 |
| 4 | Pd(OAc)2 (3) | Dioxane | - | 120 | Cs2CO3 | 14 |
| 5 | Pd(OAc)2 (3) | Toluene | - | 120 | Cs2CO3 | [b] |
| 6 | Pd(OAc)2 (3) | Toluene | PPh3 (6) | 120 | Cs2CO3 | 8 |
| 7 | Pd(OAc)2 (3) | Toluene | PPh3 (6) | 150 | Cs2CO3 | 10 |
| 8 | Pd(OAc)2 (3) | Toluene/EtOH (2:1) | - | 120 | Cs2CO3 | - |
| 9 | Pd(OAc)2 (3) | Toluene | - | 150 | Cs2CO3 | 41 |
| 10 | Pd(OAc)2 (3) | Toluene | - | 150 | K3PO4 | - |
| 11 | Pd(OAc)2 (3) | DMA | - | 150 | Cs2CO3 | [c] |
| 12 | Pd(TFA)2 (5) | Toluene | - | 120 | Cs2CO3 | 61 |
| 13 | Pd(dppf)Cl2·DCM (3) | Toluene | - | 120 | Cs2CO3 | - |
| 14 | - | Toluene | - | 150 | Cs2CO3 | 98 |
[a] Isolated yield after column chromatography. [b] 120 °C, t = 10 min. in MW 100% of starting material was recovered. [c] 150 °C, t = 1 h, MW degradation in mixture.
