Abstract
OBJECTIVE:
The pathogenesis of osteoarthritis centers on the imbalance between catabolic and anabolic processes in cartilage metabolism. Insulin growth factor 1 (IGF-1) has been shown to have anabolic effects in cartilage in vitro. This study aim to determine whether IGF-1 on cartilage is associated with loss of chondrocyte and extracellular matrix breakdown using the Hartley guinea pig model.
MATERIALS AND METHODS:
Cartilage from the medial and lateral tibial plateau of 6-month and 12-month old Hartley guinea pigs were used for this study. Histological analysis was performed with hematoxylin-eosin (HE) and toluidine blue staining. Safranin-O staining was used to quantify proteoglycan (PG) loss and the extent of cartilage damage by Modified Mankin score. Distribution of IGF-1 was demonstrated with in situ hybridization techniques. IGF-1 mRNA levels were assessed using Real-time PCR.
RESULTS:
Histological loss of chondrocytes, and cartilage matrix and decreased IGF-1 distribution were demonstrated in a temporal and spatial manner. Compared to the 6-month old samples, the 12-month specimens had significantly cartilage degeneration and less cartilage matrix and PGs staining. Decreased level of IGF-1 was also observed in the 12-month samples. These observations were more pronounced in the medial tibial plateau when compared to the lateral plateau.
CONCLUSIONS:
The decreased level of IGF-1 may play a critical role for maintaining the balance between catabolic and anabolic processes in cartilage metabolism during the development of osteoarthritis. Thus, the increase of IGF-1 may be applicable to developing OA therapy.
Keywords: Osteoarthritis, Cartilage, IGF-1, Guinea pig
Introduction
Osteoarthritis is a common affliction that debilitates millions of individuals per year. It is a disease characterized by loss of articular cartilage, hypertrophic bony changes, and inflammatory synovitis. Degenerative changes to articular cartilage are secondary to an imbalance of cartilage homeostasis in which catabolic processes outpace the reparative abilities of cartilage. Although the exact pathogenesis of osteoarthritis has not yet been elucidated, research has shown that mechanical and biochemical forces are responsible for the progression of the disease.
Insulin-like growth factor 1 (IGF-1) is a 70 amino-acid single polypeptide that shares approximately 50% homology with insulin1. It is primarily produced by the liver, although most somatic cells in the body can produce the growth factor2. Chondrocytes also release IGF-1 and can increase its production during osteoarthritic conditions3,4. The IGF-1 receptor is a specific tyrosine kinase receptor that mediates its effects via intracellular signaling. The circulating free level of IGF-1 is determined by several factors. Growth hormone secreted from the anterior pituitary induces IGF-1 production. Calcitropic hormones such as 1,25(OH)2 Vit D3 have also been shown to increase IGF-1 secretion. In osteoarthritic chondrocytes, transforming growth factor B, stimulate IGF-1 secretion5. However, the most important factor that affects IGF-1 level is the IGF binding protein (IGFBP), of which IGFBP-3 is the most important. IGF-1 binds to IGFBP-3 to form the labile acid subunit, which acts as the circulatory store of IGF-16. IGFBP-3 has been localized to pericellular matrix in articular cartilage and its level is up-regulated in osteoarthritic cartilage3,7.
The role of IGF-1 in the body is diverse. It plays critical roles in prenatal development and somatic growth; Laron dwarfism is a condition defined by chronic GH and IGF-1 deficiency that can be treated by supplementing the patient with GH/IGF-18. IGF-1 also augments both innate and acquired immunity by regulating proliferation of lymphocytes, increased TNF-a production, and enhanced NK-cell activity8. IGF-1 exerts several effects on cartilage. It induces the production of cartilage matrix components, of which PG and type II collagen are most prominent9,10.
Scholars have focused on the possible roles of cytokines and growth factors in the development of osteoarthritis. IL-1, TNF-a, and SDF-1 have all been implicated in osteoarthritic development11,12. Additional work has focused on the anabolic role of IGF-1 in cartilage metabolism. Despite the in vitro evidence supporting the protective role of IGF-1, relatively few studies have been performed in in vivo models. Furthermore, studies that attempted to correlate serum IGF-1 levels and severity of osteoarthritis have produced conflicting results13–15. Other research has demonstrated an increase of IGF-1 within the synovial fluid of osteoarthritic joints, suggesting that chondrocytes can increase its synthesis of the growth factor during periods of accelerated damage to prevent further progression of the disease16,17.
The goal of our work is to demonstrate the relationship between the level of IGF-1 and cartilage degradation in vivo. We used the Hartley guinea pig, a well-established animal model for osteoarthritis, to demonstrate that the presence of IGF-1 decreases the extent of chondrocyte and PGs loss and matrix breakdown.
Materials and Methods
Preparation of 6- and 12-Month Samples
6- and 12-month-old outbred male Hartley guinea pigs weighing 0.93±0.05 kg and 1.25±0.06 kg respectively, were used in the experiment (Charles River, Wilmington, MA, USA) N=8 in each group. The animals were killed with intraperitoneal injections of pentobarbital at 6 and 12 months age. The knee joint was harvested and the medial and lateral tibia plateaus were dissected from its soft tissue attachments. The study was approved by the Rhode Island Hospital Animal Welfare Committee (IACUC) (Approval: 0013–08).
Histological Analysis of 6- and 12-Month Samples
The knee joints from 6- and 12- month old animals were fixed overnight in 4% paraformaldehyde in phosphate-buffered saline (pH 7.4), decalcified in 10% EDTA-PBS for 1–2 weeks. Those materials were routinely processed and embedded in paraffin. Serial 5 μm sections were prepared and collected on positively charged glass slides (Superfrost Plus, Thermo Fisher Scientific, Waltham, MA, USA). The sections were dried on a hot plate to increase adherence and then stained with HE to evaluate the histological changes. The samples were stained with toluidine blue to assess proteoglycan content. Safranin-O staining was used to verify PG loss and the cartilage damage. The severity of cartilage damage of each joint was then assessed using the modified Mankin grading system18. Three independent observers scored each section and the scores for the medial and lateral tibial condyles were averaged within each joint. All scoring was done in a random order and in a blinded fashion.
The dissector method of Eggli et al19 was applied to estimate the number of chondrocytes per unit of volume tissue. Briefly, the hematoxylin and eosin stained sample was arithmetically defined into four zones. Two parallel tissue planes separated by a known distance were used. This tissue volume is called a dissector. The number of cells present in section 1 but no longer apparent in section plane 2, divided by the dissector, provided an estimate of number of cells per unit volume.
In Situ Hybridization of IGF-1
A cDNA probe encoding guinea pig IGF-1 was cloned by RT-PCR from total RNA isolated from guinea pig tibia articular cartilage using published IGF-1 mRNA primers 3’-TTCTACCTGGCCTTCTGC-5’ and 3’-GCAGTACATCTCCAGCCT-5’20. 6-month-old and 12-month-old male Hartley guinea pigs were used in this study. Eight animals from each age group were used for in situ hybridization of IGF-1 mRNA using digoxigenin UTP-labeled single-stranded RNA probes prepared with a DIG-RNA labe ling kit (Cat. No. 11 175 025 910, Roche Applied Science, Indianapolis, IN, USA). Distribution of IGF-I mRNA was detected on paraffin embedded sections.
Real-time PCR of IGF-1
Total RNA was isolated from the tibial cartilages of 6- and 12-month old animals with the RNeasy mini kit (QIAGEN, Valencia, CA, USA) as previously described by Wei et al21. 1 μg total of RNA was used for each reverse transcriptase with the iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). Real-time quantitative PCR amplification was performed using QuantiTect SYBR Green PCR kit (QIAGEN, Valencia, CA, USA) with the DNA Engine Opticon 2 Continuous Fluorescence Detection System (MJ Research, Waltham, MA, USA). Primers used in amplification of target genes’ mRNA are: 3’-TTCTACCTGGCCTTCTGC-5’ and 3’-GCAGTACATCTCCAGCCT-5’. IGF-1 mRNA level was normalized to housekeeping gene 18S RNA levels. Since the level of 18S RNA is constant in all the cells, the normalized values reflected the relative level of the target genes’ mRNA in each cell regardless of the cell number. The 18S RNA was amplified at the same time and used as an internal control. The cycle threshold (Ct) values for 18S RNA and that of samples were measured and calculated by computer software (MJ Research, Waltham, MA, USA). Relative transcription levels were calculated as x = −2ΔΔCt, in which Ct = E - C, and E = Ctexp-Ct18s; C = Ctctl-Ct18s.
Statistical Analysis
SPSS 19.0 software (IBM, Armonk, NY, USA) was used for statistical analysis. All quantitative data were expressed as mean ± standard deviation. Student t-test was used to analyze the comparisons between 6- and 12-months groups. p values < 0.05 were considered statistically significant
Results
Chondrocyte Number and Matrix Integrity
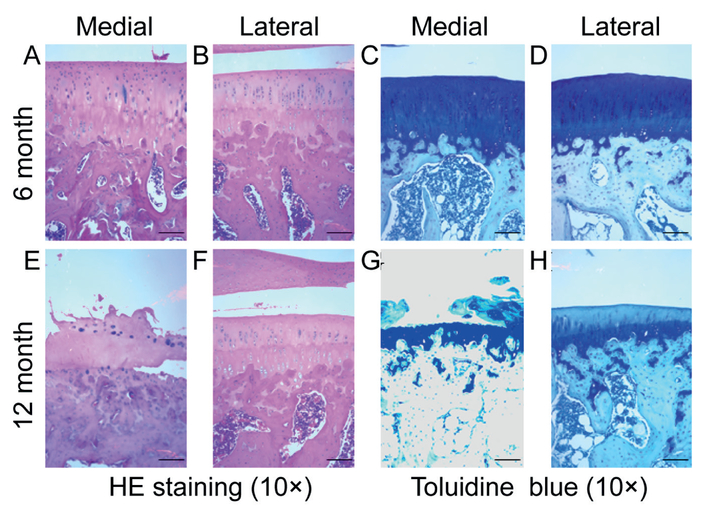
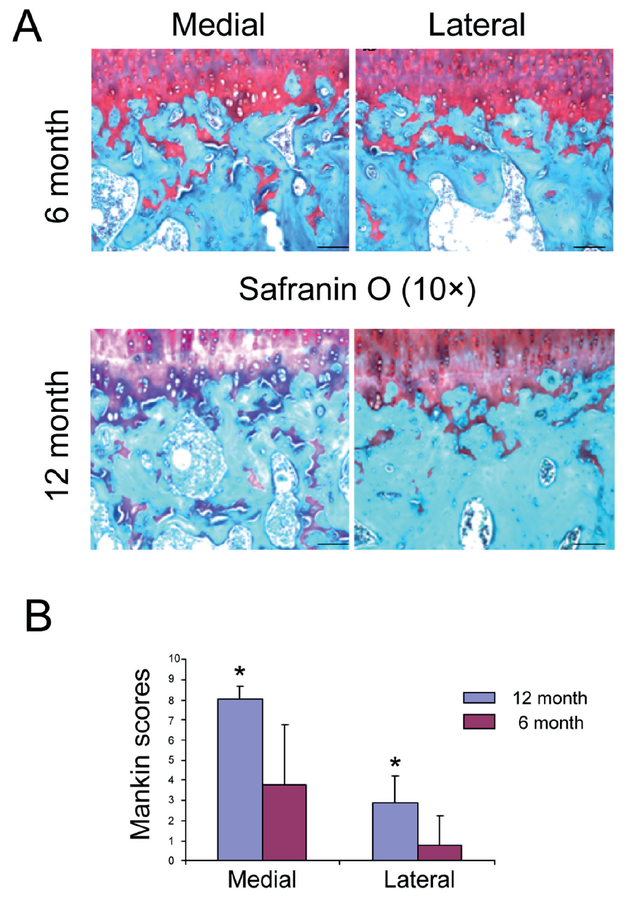
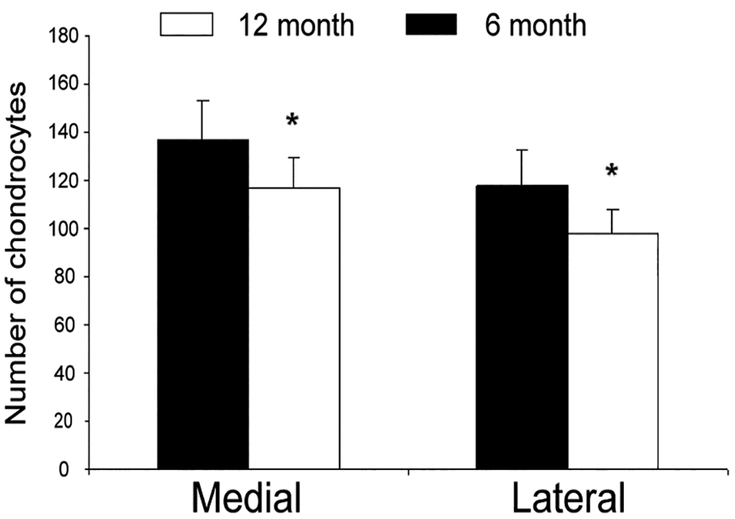
Histological analysis of paraffin-embedded samples from the tibial plateau demonstrated decreased cellularity and matrix integrity in a temporal and spatial-dependent manner. The extent of cartilage disruption and proteoglycan loss was more severe in the medial plateau and in the 12-month sample (Figure 1). Safranin O staining revealed an increase in cartilage damage and Mankin grade corresponded to a decrease in PGs staining (Figure 2). Compared to the six-month old samples, there was a significant decrease in chondrocyte number in both medial and lateral tibial plateau of 12-month old samples (Figure 3).
Figure 1.
Histological analysis of 6 and 12 months cartilage. HE and toludine blue staining was used to assess chondrocyte and proteoglycan loss. (A-B): There is minimal cell and matrix disruption in the medial and lateral plateau of the 6 months cartilage. (E-H): In the medial plateau of the 12 months cartilage, there is a cartilage destruction with the crack between calcified and uncalcified cartilage interface, chondrocyte loss, and matrix breakdown with loss of proteoglycans. Even with the intact articular surface, there is a loss of chondrocytes and matrix components in the lateral plateau of 12 month samples. Scale bar = 200 μm.
Figure 2.
Safranin O staining and Mankin grading (A) Safranin O staining and (B) the modified Mankin score showed a marked increase in cartilage damage for the 12 month knees at the medial side as compared to the 6-month joints. Loss of proteoglycan staining and chondrocytes and cartilage destruction were evident in the 12-month-old animals. Scale bar = 200 μm.
Figure 3.
Quantification of chondrocytes in 6 and 12 month cartilage. Light microscopy was used to count the number of chondrocytes in 6 and 12-month cartilage samples. The number of chondrocytes was higher in the 6 month cartilage, with loss of cellularity observed in the 12 month cartilage. This pattern was observed in both the medial and lateral plateau. Bar graphs show the averages of quantified data from 8 animals. *: Statistically significant in comparison to 6-month-old animals, p<0.05.
In Situ Distribution of IGF-1
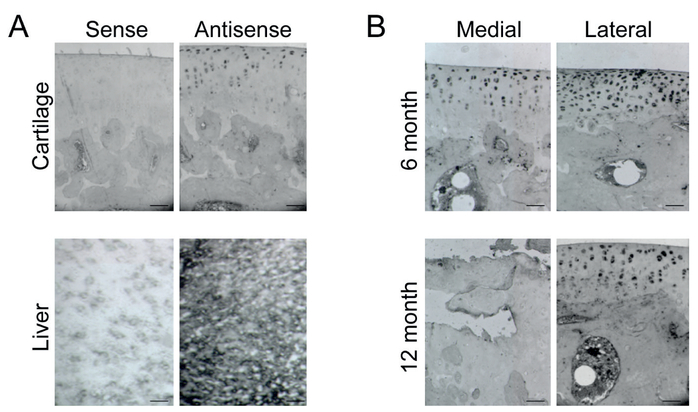
In situ hybridization of IGF-1 using single stranded DIG-UTP-labeled sense and antisense riboprobe demonstrated decreased IGF-1 levels in a temporal and spatial -dependent manner. The primary localization of IGF-1 is within the superficial zone of cartilage. The extent of IGF-1 loss was more severe in the medial plateau and in the 12-month sample. Liver tissue served as the positive control and sense probe served as the negative control for IGF-1 hybridization (Figure 4).
Figure 4.
In situ distribution of IGF-1: A, Positive and Negative control of DIG-UTP IGF-1 insitu hybridization. Negative and positive controls of in situ hybridization on paraffin embedded sections of cartilage and frozen section of liver with single-stranded DIG-UTP-labeled sense and antisense riboprobes; B, In situ hybridization of IGF-1 in 6 and 12 month cartilage. The distribution of IGF-1 was detected with in situ hybridization on paraffin embedded sections with single-stranded DIG-UTP-labelled antisense riboprobe. IGF-1 was distributed mainly in uncalcified cartilage. In the 6 month sample, IGF-1 is expressed in the superficial cartilage and that its expression was more pronounced in the lateral plateau of cartilage. In the 12 month sample, there is obvious cartilage destruction with minimal IGF-1 expression in the medial plateau. In the same sample, the cartilage structure is intact in the lateral plateau, but there is decreased IGF-1 expression when compared to the 6month lateral plateau. Scale bar = 100 μm.
IGF-1 mRNA Levels
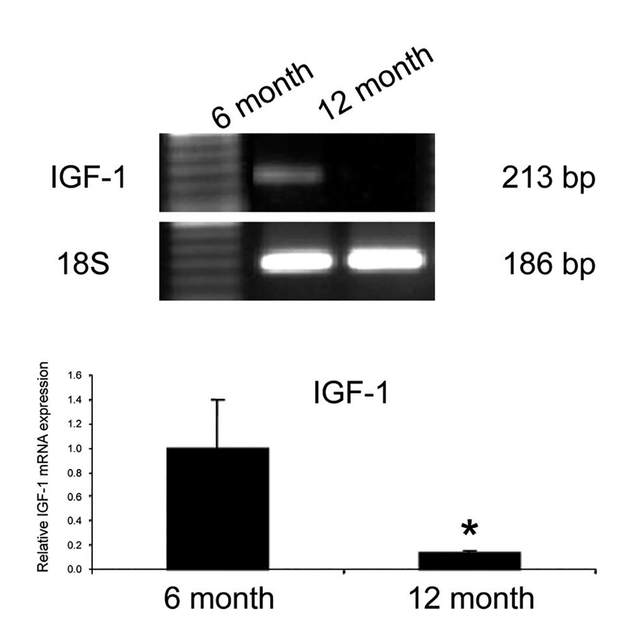
Real-time PCR demonstrated significantly decreased levels of IGF-1 mRNA in 12-month old cartilage samples compared to 6-month old samples. Results from Real-time PCR confirmed this finding, demonstrating that the level of IGF-1 mRNA in the 12-month old cartilage sample was five times less compared to levels found in the 6-month old sample. 18S served as the internal control (Figure 5).
Figure 5.
mRNA levels of IGF-1 in 6 and 12 month cartilage: Real-time PCR was used to quantify mRNA levels of IGF-1 in 6 and 12 month old cartilage. Using 18S as the internal control, there was decreased IGF-1 mRNA in 12 month old sample. Bar graphs show the averages of quantified data of IGF-1 using Real-time PCR from three independent experiments. *: Significant difference in comparison to 6-month-old animals, p<0.05.
Discussion
The exact pathogenesis of osteoarthritis has not been clearly defined. However, a combination of mechanical and biochemical factors play significant roles in the development of the disease. Increasing age and mechanical wear have been linked to osteoarthritic changes in cartilage. Scholars have focused on the role of biochemical factors, such as cytokines, chemokines, and growth factors in osteoarthritis. Inflammatory cytokines such as IL-1 and TNF-a have been associated with osteoarthritis. Chemokine molecules such as SDF-1 stimulate MMP-mediated cartilage degradation22,23. There is a balance between anabolic and catabolic factors that help maintain cartilage homeostasis. Chondrocyte is the only cell to maintain the balance in the cartilage. Insulin growth factor-1 plays a critical role in cartilage anabolism and has been associated with production of cartilage matrix proteins24 and cell proliferation25. However, there is no directive correlation between IGF-1 and OA cartilage degradation. In this study, we show a decrease of IGF-1 level is associated with loss of chondrocyte and PGs and cartilage damage.
With the onset of cartilage damage, reparative processes are initiated, as chondrocytes begin to synthesize components that are used to rebuild the matrix cell proliferation26,27. This may explain why IGF-1 levels are initially elevated in synovial28,29. The chondrocytes, in response to increasing damage, produce more IGF-1 to augment the production of matrix proteins that are essential to the reparative process. IGF-1 induces the synthesis of collagen II and proteoglycans24, two components integral to cartilage structure and function. However, if sufficient cartilage loss has occurred and IGF-1 levels are decreased, the chondrocyte’s reparative abilities are reduced. The rate of cartilage destruction outpaces the repair, leading to the progression of osteoarthritis, while there is significant in vitro data supporting the protective role of IGF-1 in chondrocyte survive and proliferation25. Okadaic acid (OA)-induced apoptosis was significantly decreased by pre-treatment with 10 ng/ml of IGF-1 for 24 h30. However, the studies demonstrating in vivo evidence are limited. We have used the Hartley guinea pig because it is a consistent animal model for osteoarthritis31. Osteoarthritic changes become more pronounced in older animals32. Furthermore, the loading forces at the knee predispose the medial compartment to earlier and more severe degenerative changes33.
In this study, we have demonstrated a clear association between the onset and severity of osteoarthritis with IGF-1 distribution and level in the local cartilage environment. Our morphologic analysis confirms that the medial compartment of the knee is more severely affected in osteoarthritis, with significant loss of chondrocytes, PGs, matrix, and overall structure (Figure 1 and 3). We also demonstrate that the severity of degeneration increases with age (Figure 2).
To illustrate the association between severity of osteoarthritis and IGF-1 levels, we have utilized in situ hybridization techniques to identify in vivo expression of IGF-1. Because the liver is the primary source of IGF-1 synthesis, we used it as our positive control (Figure 4A). We demonstrate decreased IGF-1 levels in the medial compartment of knee cartilage and in the 12-month cartilage samples. With increasing severity of osteoarthritis and subsequent loss of chondrocytes, PGs, and matrix integrity, a decline in IGF-1 production is observed (Figure 4B). This absolute loss of IGF-1 is associated with diminished IGF-1 production, as the expression of IGF-1 mRNA is reduced in the 12-month old sample (Figure 5). As a result, the surviving chondrocytes are less capable of synthesizing important matrix components that can adequately repair the damage. This is consistent with other lab’s result. In a rat genetic mutation model, IGF-1 deficiency causes an increased severity of articular cartilage lesions of OA34. The compromised structural integrity of the cartilage matrix reduces its functional ability to handle increasing mechanical stress. This forms a destructive cycle that allows the continued and complete destruction of articular cartilage in osteoarthritis.
Several studies35 have demonstrated an age-dependent increase in IGFBP-3 expression in osteoarthritic cartilage. Although it is possible that the decrease in IGF-1 distribution in the medial plateau of 12-month old cartilage is attributed to increased IGFBP-3, we demonstrate an independent decrease in the synthesis of IGF-1 by chondrocytes that exacerbates the relative decrease in free IGF-1 levels in cartilage.
Conclusions
The pathogenesis of osteoarthritis centers on mechanical and biochemical factors. Its progression is dependent upon the balance between catabolic and anabolic processes. IGF-1 plays an important and anabolic role; therefore, it serves as a protective role in osteoarthritis. We provide in vivo evidence that IGF-1 reduces the loss of chondrocytes and matrix integrity and is a viable target for novel therapeutic options for the management of osteoarthritis.
Acknowledgments
This project was supported by NIH AR052479 and 1R01AR059142–01A1, and by grants from the Aircast Foundation, Arthritis National Research Foundation, NSF 30872616, and SXNSF 2011011042. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. We would like to thank Suzanne Smith for her help in editing the manuscript.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
References
- 1).Stewart AF. PTHrP(1–36) as a skeletal anabolic agent for the treatment of osteoporosis. Bone 1996; 19: 303–306. [DOI] [PubMed] [Google Scholar]
- 2).Escribano O, Guillen C, Nevado C, Gomez-Hernandez A, Kahn CR, Benito M. Beta-Cell hyperplasia induced by hepatic insulin resistance: role of a liver-pancreas endocrine axis through insulin receptor a isoform. Diabetes 2009; 58: 820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Olney RC, Tsuchiya K, Wilson DM, Mohtai M, Maloney WJ, Schurman DJ, Smith RL. Chondrocytes from osteoarthritic cartilage have increased expression of insulin-like growth factor I (IGF-I) and IGF-binding protein-3 (IGFBP-3) and −5, but not IGF-II or IGFBP-4. J Clin Endocrinol Metab 1996; 81: 1096–1103. [DOI] [PubMed] [Google Scholar]
- 4).Tesch GH, Handley CJ, Cornell HJ, Herington AC. Effects of free and bound insulin-like growth factors on proteoglycan metabolism in articular cartilage explants. J Orthop Res 1992; 10: 14–22. [DOI] [PubMed] [Google Scholar]
- 5).Elford PR, Lamberts SW. Contrasting modulation by transforming growth factor-beta-1 of insulin-like growth factor-I production in osteoblasts and chondrocytes. Endocrinology 1990; 127: 1635–1639. [DOI] [PubMed] [Google Scholar]
- 6).Baxter RC. IGF binding protein-3 and the acid-labile subunit: formation of the ternary complex in vitro and in vivo. Adv Exp Med Biol 1993; 343: 237–244. [DOI] [PubMed] [Google Scholar]
- 7).Martin JA, Buckwalter JA. The role of chondrocyte-matrix interactions in maintaining and repairing articular cartilage. Biorheology 2000; 37: 129–140. [PubMed] [Google Scholar]
- 8).Heemskerk VH, Daemen MA, Buurman WA. Insulin-like growth factor-1 (IGF-1) and growth hormone (GH) in immunity and inflammation. Cytokine Growth Factor Rev 1999; 10: 5–14. [DOI] [PubMed] [Google Scholar]
- 9).Guenther HL, Guenther HE, Froesch ER, Fleisch H. Effect of insulin-like growth factor on collagen and glycosaminoglycan synthesis by rabbit articular chondrocytes in culture. Experientia 1982; 38: 979–981. [DOI] [PubMed] [Google Scholar]
- 10).McQuillan DJ, Handley CJ, Campbell MA, Bolis S, Milway VE, Herington AC. Stimulation of proteoglycan biosynthesis by serum and insulin-like growth factor-I in cultured bovine articular cartilage. Biochem J 1986; 240: 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Wei L, Sun X, Kanbe K, Wang Z, Sun C, Terek R, Chen Q. Chondrocyte death induced by pathological concentration of chemokine stromal cell-derived factor-1. J Rheumatol 2006; 33: 1818–1826. [PubMed] [Google Scholar]
- 12).Kim YS, Bigliani LU, Fujisawa M, Murakami K, Chang SS, Lee HJ, Lee FY, Blaine TA. Stromal cell-derived factor 1 (SDF-1, CXCL12) is increased in subacromial bursitis and downregulated by steroid and nonsteroidal anti-inflammatory agents. J Orthop Res 2006; 24: 1756–1764. [DOI] [PubMed] [Google Scholar]
- 13).Denko CW, Boja B, Moskowitz RW. Growth promoting peptides in osteoarthritis: Insulin, insulin-like growth factor-1, growth hormone. J Rheumatol 1990; 17: 1217–1221. [PubMed] [Google Scholar]
- 14).Hochberg MC, Lethbridge-Cejku M, Scott WJ, Reichle R, Plato CC, Tobin JD. Serum levels of insulin-like growth factor in subjects with osteoarthritis of the knee. Data from the Baltimore Longitudinal Study of Aging. Arthritis Rheum 1994; 37: 1177–1180. [DOI] [PubMed] [Google Scholar]
- 15).Xia M, Li H, Wang JJ, Zeng HJ, Wang SH. MiR-99a suppress proliferation, migration and invasion through regulating insulin-like growth factor 1 receptor in breast cancer. Eur Rev Med Pharmacol Sci 2016; 20: 1755–1763. [PubMed] [Google Scholar]
- 16).Schneiderman R, Snir E, Popper O, Hiss J, Stein H, Maroudas A. Insulin-like growth factor-I and its complexes in normal human articular cartilage: studies of partition and diffusion. Arch Biochem Biophys 1995; 324: 159–172. [DOI] [PubMed] [Google Scholar]
- 17).Tavera C, Abribat T, Reboul P, Dore S, Brazeau P, Pelletier JP, Martel-Pelletier J. IGF and IGF-binding protein system in the synovial fluid of osteoarthritic and rheumatoid arthritic patients. Osteoarthritis Cartilage 1996; 4: 263–274. [DOI] [PubMed] [Google Scholar]
- 18).van der Sluijs JA, Geesink RG, van der Linden AJ, Bulstra SK, Kuyer R, Drukker J. The reliability of the Mankin score for osteoarthritis. J Orthop Res 1992; 10: 58–61. [DOI] [PubMed] [Google Scholar]
- 19).Eggli PS, Hunziker EB, Schenk RK. Quantitation of structural features characterizing weight- and less-weight-bearing regions in articular cartilage: a stereological analysis of medial femoral condyles in young adult rabbits. Anat Rec 1988; 222: 217–227. [DOI] [PubMed] [Google Scholar]
- 20).Gosiewska A, Wilson S, Kwon D, Peterkofsky B. Evidence for an in vivo role of insulin-like growth factor-binding protein-1 and −2 as inhibitors of collagen gene expression in vitamin C-deficient and fasted guinea pigs. Endocrinology 1994; 134: 1329–1339. [DOI] [PubMed] [Google Scholar]
- 21).Wei L, Sun XJ, Wang Z, Chen Q. CD95-induced osteoarthritic chondrocyte apoptosis and necrosis: Dependency on p38 mitogen-activated protein kinase. Arthritis Res Ther 2006; 8: R37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Shui XL, Lin W, Mao CW, Feng YZ, Kong JZ, Chen SM. Blockade of IL-17 alleviated inflammation in rat arthritis and MMP-13 expression. Eur Rev Med Pharmacol Sci 2017; 21: 2329–2337. [PubMed] [Google Scholar]
- 23).Kanbe K, Takemura T, Takeuchi K, Chen Q, Takagishi K, Inoue K. Synovectomy reduces stromal-cell-derived factor-1 (SDF-1) which is involved in the destruction of cartilage in osteoarthritis and rheumatoid arthritis. J Bone Joint Surg Br 2004; 86: 296–300. [DOI] [PubMed] [Google Scholar]
- 24).Chubinskaya S, Hakimiyan A, Pacione C, Yanke A, Rappoport L, Aigner T, Rueger DC, Loeser RF. Synergistic effect of IGF-1 and OP-1 on matrix formation by normal and OA chondrocytes cultured in alginate beads. Osteoarthritis Cartilage 2007; 15: 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Massicotte F, Aubry I, Martel-Pelletier J, Pelletier JP, Fernandes J, Lajeunesse D. Abnormal insulin-like growth factor 1 signaling in human osteoarthritic subchondral bone osteoblasts. Arthritis Res Ther 2006; 8: R177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Wei L, Svensson O, Hjerpe A. Proteoglycan turnover during development of spontaneous osteoarthrosis in guinea pigs. Osteoarthritis Cartilage 1998; 6: 410–416. [DOI] [PubMed] [Google Scholar]
- 27).Zhao XX, Bi Y, Yin XY, Min R. Suppression of collagen-induced arthritis by lipopolysaccharide in DBA/1 mice. Eur Rev Med Pharmacol Sci 2016; 20: 441–446. [PubMed] [Google Scholar]
- 28).Lis K, Odrowaz-Sypniewska G, Nowacki W. [Evaluation of IGF-1 concentration in serum and synovial fluid in women with different type coxarthrosis]. Chir Narzadow Ruchu Ortop Pol 2005; 70: 407–410. [PubMed] [Google Scholar]
- 29).Vasara AI, Konttinen YT, Peterson L, Lindahl A, Kiviranta I. Persisting high levels of synovial fluid markers after cartilage repair: a pilot study. Clin Orthop Relat Res 2009; 467: 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Xing C, Peng Y, Chang R, Yin Y, Xie Z. Effects of insulin-like growth factor-1 on okadaic acid-induced apoptosis in SH-SY5Y cells. Cell Biol Int 2005; 29: 803–808. [DOI] [PubMed] [Google Scholar]
- 31).Huebner JL, Seifer DR, Kraus VB. A longitudinal analysis of serum cytokines in the Hartley guinea pig model of osteoarthritis. Osteoarthritis Cartilage 2007; 15: 354–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).de Bri E, Lei W, Reinholt FP, Mengarelli-Widholm S, Heingard D, Svensson O. Ultrastructural immunolocalization of bone sialoprotein in guinea-pig osteoarthritis. Osteoarthritis Cartilage 1997; 5: 387–393. [DOI] [PubMed] [Google Scholar]
- 33).Brismar BH, Lei W, Hjerpe A, Svensson O. The effect of body mass and physical activity on the development of guinea pig osteoarthrosis. Acta Orthop Scand 2003; 74: 442–448. [DOI] [PubMed] [Google Scholar]
- 34).Ekenstedt KJ, Sonntag WE, Loeser RF, Lindgren BR, Carlson CS. Effects of chronic growth hormone and insulin-like growth factor 1 deficiency on osteoarthritis severity in rat knee joints. Arthritis Rheum 2006; 54: 3850–3858. [DOI] [PubMed] [Google Scholar]
- 35).De Ceuninck F, Caliez A, Dassencourt L, Anract P, Renard P. Pharmacological disruption of insulin-like growth factor 1 binding to IGF-binding proteins restores anabolic responses in human osteoarthritic chondrocytes. Arthritis Res Ther 2004; 6: R393–R403. [DOI] [PMC free article] [PubMed] [Google Scholar]