Abstract
Objective
This study used a decision-analytic framework to assess the cost-effectiveness of brexpiprazole vs comparator branded therapies for reducing relapses and hospitalizations among adults with schizophrenia from a US payer perspective.
Methods
An economic model was developed to assess patients with stable schizophrenia initiating treatment with brexpiprazole (1–4 mg), cariprazine (1–6 mg), or lurasidone (40–80 mg) over a 1-year period. After 6 months, patients remained on treatment or discontinued due to relapse, adverse events, or other reasons. Patients who discontinued due to relapse or adverse events were assumed to have switched to other therapy, and those who discontinued due to other reasons were assumed to have received no therapy. Primary outcomes were incremental cost per relapse avoided and hospitalization avoided, and the secondary outcome was cost per quality-adjusted life-year (QALY) gained. Sensitivity and scenario analyses were also conducted.
Results
Brexpiprazole was associated with the highest per-patient clinical effectiveness (avoided relapses 0.637, avoided hospitalizations 0.719, QALYs 0.707) among comparators, followed by cariprazine (avoided relapses 0.590, avoided hospitalizations 0.683, QALYs 0.683) and lurasidone (avoided relapses 0.400, avoided hospitalizations 0.536, QALYs 0.623). Annual per-patient health-care costs were lowest for brexpiprazole ($20,510), followed by cariprazine ($22,282) and lurasidone ($25,510). Brexpiprazole was the least costly and most effective treatment strategy for all outcomes. Results were sensitive to relapse rates and daily cost of brexpiprazole. Limitations include data principally obtained from drug-specific randomized withdrawal studies and lack of direct-comparison trials.
Conclusion
This analysis evaluated brexpiprazole treatment for the reduction of schizophrenia relapses and hospitalizations over a 1-year period compared to other recently available branded antipsychotics, and excluded generic antipsychotic treatments. Brexpiprazole treatment may lead to clinical benefits and medical cost savings, and provides a cost-effective treatment option for patients relatively to other branded second-generation antipsychotics.
Keywords: schizophrenia, cost-effectiveness, relapse prevention, cost-benefit, indirect analysis, event avoided, hospitalization avoided, brexpiprazole
Introduction
Schizophrenia is a complex and disabling mental disorder characterized by delusions, hallucinations, disorganized speech and behavior, negative symptoms, cognitive impairment, and other symptoms that contribute to social and occupational dysfunction.1 The disorder affects approximately 1.1% of adults in the USA.2 The economic burden of schizophrenia in the US is substantial: estimated at $156 billion in 2013.3 Schizophrenia-relapse rates are high, and further contribute to the economic burden of the disorder. Acher-Svanum et al found that total annual direct mental health-care costs were about three times higher among persons with schizophrenia who experienced relapses in the 6 months prior to the study compared to patients who did not experience relapses in that period.4
The goals of schizophrenia treatment have evolved over the last several decades, and focus on increasing quality of life (QoL) and functioning and striving for remission.5–7 Guidelines recommend psychosocial interventions incorporated into all phases of patient management, with the goal of minimizing stress and maximizing patient functioning.6 The American Psychiatric Association practice guidelines support the use of programs, such as community interventions (eg, Program for Assertive Community Treatment, family interventions, supported employment, cognitive behavioral therapy, social skills training, and programs of early intervention to delay relapse).6 The association recommends antipsychotic agents as the mainstay of treatment for schizophrenia; however, variance in pharmacological profiles create clinically relevant variability in tolerability and efficacy.8 Typical antipsychotics (ie, first-generation antipsychotics) are antagonists at dopamine D2 receptors and effective against psychotic symptoms. However, these agents have a high rate of motoric adverse events (AEs), such as drug-induced parkinsonism and tardive dyskinesia, at therapeutic doses. Atypical antipsychotic agents (ie, second-generation antipsychotics [SGAs]) available in the US include clozapine, risperidone, olanzapine, quetiapine, ziprasidone, aripiprazole, lurasidone, paliperidone, iloperidone, cariprazine, and asenapine. These atypical antipsychotics differ from one another in their tolerability profile. Although atypical antipsychotics may have reduced risk of motoric side effects, there is evidence that demonstrates variable risk of weight gain, diabetes, hyperlipidemia, and cardiovascular complications.
Although antipsychotic medications can manage the symptoms of schizophrenia effectively and help patients to achieve remission, relapses are common.9 In addition, discontinuation rates for antipsychotic medications are high in both clinical trial and real-world settings. For example, in the US Clinical Antipsychotic Trials of Intervention Effectiveness study,10 the overall discontinuation rate over 18 months for patients with chronic schizophrenia taking antipsychotics was 74%, and in a 3-year European observational study discontinuation rates were 34%–66%.11
Poor tolerability and side effects of antipsychotics are among the primary reasons for premature treatment discontinuation, resulting in inadequate symptom resolution and an increased risk of relapse.12,13 Therefore, additional tolerable treatment options are needed. Brexpiprazole is an SGA approved by the US Food and Drug Administration (FDA) in July 2015 as monotherapy for schizophrenia in adults. The efficacy of brexpiprazole in schizophrenia was demonstrated in two 6-week, randomized, double-blind, placebo-controlled, fixed-dose clinical trials.14,15 Brexpiprazole has demonstrated a low incidence of sedating or activating AEs, a low rate of long-term metabolic effects, and moderate weight gain.14–16 The efficacy of brexpiprazole was also demonstrated in a randomized-withdrawal, double-blind, placebo-controlled, 52-week maintenance study,17 which showed a reduction in risk of relapse of 71% vs placebo over 1 year and an incidence of AEs that was comparable to placebo.
Evidence of efficacy and tolerability remains important in the evaluation and comparison of available therapies; however, it is also important to determine their cost-effectiveness, given limitations on health-care spending. In the absence of head-to-head studies, indirect comparisons to evaluate the cost-effectiveness of treatments for schizophrenia can assist in health-care decision-making. This study used a decision-analytic framework to assess the cost-effectiveness of brexpiprazole in schizophrenia for reducing relapses and hospitalizations among adults with schizophrenia from a US payer perspective.
Evaluating the cost-effectiveness of newly available branded treatments in schizophrenia can shape policies concerning treatment coverage and reimbursement. Decision makers emphasize the need for more timely information.18 In the US, the majority of oral antipsychotics are available as generic products. Given increasing pressures to manage health-care costs, it is expected that generic-drug utilization is generally prioritized over the use of branded treatments. For policy makers evaluating new branded treatments for formulary placement, an appropriate pharmacoeconomic analysis would involve comparisons of newly available and existing branded treatments.
Methods
Model overview
A decision-analytic model was developed to evaluate a hypothetical cohort of adult patients with stable schizophrenia initiating treatment with brexpiprazole, cariprazine, or lurasidone. Lurasidone and cariprazine were selected as comparators because they are the most recently FDA-approved SGAs and long-term prevention studies on them were comparable to those on brexpiprazole in terms of patient population, trial design, and end points. In the long-term maintenance trials, all patients were stabilized before entering a randomized, double-blind phase for at least 12 weeks. Additionally, relapse definitions were comparable across trials. Modeled treatment doses were based on those evaluated in long-term prevention studies from which clinical events were derived: brexpiprazole (1–4 mg), cariprazine (1–6 mg), and lurasidone (40–80 mg). It was assumed that patients remained adherent to treatment during treatment-initiation and -switch periods.
Model outputs are reported over a 1-year model time horizon, which was chosen to be consistent with the clinical trial duration period. It is clinically relevant to model schizophrenia outcomes within 1 year, because clinical effectiveness and treatment discontinuation are typically seen within this period and data beyond 1 year are limited.10,19 Due to the length of the time horizon, discounting (ie, translating future costs and benefits into present-day values) was not applied.
The model incorporated direct costs related to drug acquisition, AE treatment, relapse-related treatment, and patient monitoring. All costs are reported in 2016 US$. Clinical events were estimated from long-term relapse trials (efficacy) and acute trials (AEs) for each model comparator. Cost-effectiveness was evaluated for primary outcomes of incremental cost per relapse avoided and cost per hospitalization avoided and the secondary outcome of cost per quality-adjusted life-year (QALY) gained. The model was programmed using Microsoft Excel 2010.
Model population and structure
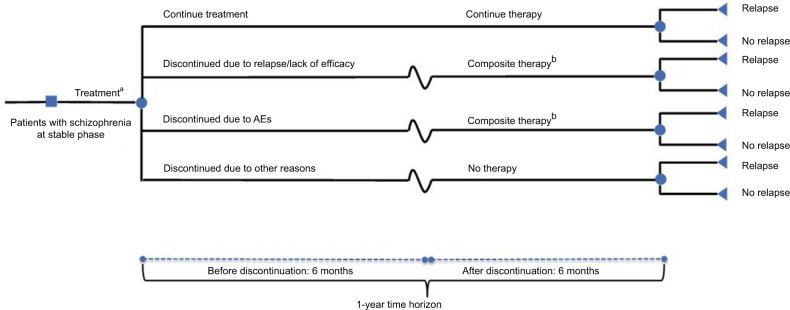
The model evaluated a hypothetical cohort of adults with stable- phase schizophrenia consistently with patients enrolled in long-term prevention studies of brexpiprazole,17 cariprazine,20 and lurasidone.21 Initially, patients entered the model and were treated with brexpiprazole, cariprazine, or lurasidone (Figure 1). Following treatment initiation, patients remained on therapy for the full year or discontinued treatment after 6 months due to relapse/lack of treatment efficacy, AEs, or other reasons (including cost of medication, nonadherence, patient preference, or unknown). Because the median time to discontinuation in the brexpiprazole study17 was 169 days, the use of 6 months (approximately 183 days) was unlikely to bias model results.
Figure 1.
Model structure.
Notes: aTreatment included brexpiprazole, lurasidone, and cariprazine; bComposite therapy: olanzapine, risperidone, quetiapine, ziprasidone, aripiprazole
Abbreviation: AEs, adverse events.
Patients who discontinued due to relapse/lack of treatment efficacy or AEs were assumed to switch to composite therapy, which included generic SGAs (ie, olanzapine, risperidone, quetiapine, ziprasidone, and aripiprazole). Patients who discontinued due to other reasons were assumed to receive no additional therapy. For patients who switched to composite therapy, it was assumed that the likelihood of receiving any one of the therapies was the same. Therefore, rates of relapse and AEs were calculated as averages for the composite therapies. Although patients who switched to composite therapy could experience relapse or AEs, they were assumed to continue treatment throughout the remainder of the year.
Model estimation
Clinical inputs
Key clinical inputs were derived from 52-week maintenance relapse studies for brexpiprazole17 and cariprazine,20 a 28-week maintenance relapse study for lurasidone,21 and published reports and package inserts (PIs) for SGAs used for composite therapy. Clinical parameters included in the model were rates of treatment discontinuation, relapse/impending relapse, and AEs (Tables 1–3).
Table 1.
Probability of treatment discontinuation and relapse at 6 months
| Mean | SEa | Source | |
|---|---|---|---|
| Probability of treatment discontinuation at 6 months due to relapse | |||
| Brexpiprazole | 16.0% | 0.8% | Fleischhacker et al17 |
| Lurasidone | 30.9% | 1.5% | Tandon et al21 |
| Cariprazine | 18.5% | 0.9% | Durgam et al20 |
| Probability of treatment discontinuation at 6 months due to AEs | |||
| Brexpiprazole | 23.3% | 1.2% | Fleischhacker et al17 |
| Lurasidone | 7.8% | 0.4% | Tandon et al21 |
| Cariprazine | 8.2% | 0.4% | Durgam et al20 |
| Probability of treatment discontinuation at 6 months due to other reasonsb | |||
| Brexpiprazole | 16.5% | 0.8% | Fleischhacker et al17 |
| Lurasidone | 32.0% | 1.6% | Tandon et al21 |
| Cariprazine | 18.8% | 0.9% | Durgam et al20 |
| Probability of relapse in composite arm | |||
| Composite therapy | 14.9%b | NA | Calculation |
| Olanzapine | 4.0% | 0.2% | Beasley et al43 |
| Risperidone | 15.0% | 0.8% | Csernansky et al39 |
| Quetiapine | 15.4% | 0.8% | Peuskens et al44 |
| Ziprasidone | 16.3% | 0.8% | Arato et al45 |
| Aripiprazole | 23.7% | 1.2% | Pigott et al46 |
Notes:
All SEs assumed to be 5% of the mean;
other reasons included cost of medication, nonadherence, patient preference, or unknown.
Abbreviation: NA, not applicable.
Table 2.
Adverse-event rates
| Treatment | Akathisia, mean (SE) | EPS, mean (SE) | Glucose abnormalities,a mean (SE) | Lipid abnormalities,b mean (SE) | Sedation, mean (SE) | Weight gain ≥7%, mean (SE) | Source |
|---|---|---|---|---|---|---|---|
| Brexpiprazole | 6.9% (0.3%) | 14.0% (0.7%) | 3.4% (0.2%) | 2.3% (0.1%) | 2.7% (0.1%) | 10.2% (0.5%) | Rexulti PI24 |
| Lurasidone | 11.5% (0.6%) | 11.5% (0.6%) | 9.6% (0.5%) | 5.7% (0.3%) | 10.0% (0.5%) | 4.8% (0.2%) | Latuda PI25 Citrome26 |
| Cariprazine | 11.0% (0.6%) | 16.0%c (0.8%) | 8.4% (0.4%) | 3.4%d (0.2%) | 4.0% (0.2%) | 3.0% (0.2%) | Vraylar summary review47 Durgam et al48 |
| Composite therapy | 7.2% (0.4%) | 14.0% (0.7%) | 5.3% (0.3%) | 6.4% (0.3%) | 15.4% (0.8%) | 14.8% (0.7%) | Calculation (mean rate across SGAs) |
| Olanzapine | 8.7% | 24.2% | 2.2% | 2.8% | 29.9%e | 22.2% | Zyprexa PI49 |
| Risperidone | 10.0% | 15.1%f | 0.3% | 4.6% | 8.2% | 10.8% | Risperdal PI50 |
| Quetiapine | 1.2% | 3.5%g | 2.4% | 18.0% | 18.0%e | 23.0% | Seroquel PI51 |
| Ziprasidone | 8.0% | 14.0% | 17.6% | 3.9% | 14.0%e | 9.7% | Geodon PI52 |
| Aripiprazole | 8.0% | 13.0% | 3.8% | 2.5% | 7.0%h | 8.1% | Abilify PI53 |
Notes: Absolute rates (not placebo-adjusted).
Fasting glucose criteria: normal to high (<100–≥126 mg/dL). For lurasidone, the normal to high criterion was ≥160 mg/dL; for risperidone, the normal–high cutoff was <140 mg/dL to ≥200 mg/dL.
Fasting total cholesterol criteria: normal–high (<200–≥240 mg/dL). For lurasidone, the normal–high criterion was ≤200 mg/dL; for quetiapine, the criterion was ≥240 mg/dL.
Any EPS, excluding akathisia/restlessness.
Weighted average calculated based on total cholesterol (>1.3 × ULN [200 mg/dL]) rates from three treatment arms: 1.5, 3, and 4.5 mg.
No sedation data reported. Somnolence rate was used.
No EPS data reported. Parkinsonism (includes extrapyramidal disorder, musculoskeletal stiffness, parkinsonism, cogwheel rigidity, akinesia, bradykinesia, hypokinesia) rate from two treatment arms: 2–8 mg and >8–16 mg/day.
Includes restless and extrapyramidal disorder.
Data from pooled incidence (rounded) of adverse reactions that occurred during acute therapy (up to 6 weeks in schizophrenia and up to 3 weeks in bipolar mania).
Abbreviations: EPS, extrapyramidal symptoms; PI, package insert; SGAs, second-generation antipsychotics; ULN, upper limit of normal.
Table 3.
Estimated cost inputs
| Estimate | SE | Source | |
|---|---|---|---|
| Treatment-related costs | |||
| Monitoring | $95.19 | $4.76 | US Bureau of Labor Statistics27 |
| Treatment switch | $282.98 | $14.15 | Citrome et al19 |
| Relapse-treatment costs | |||
| Inpatient | $32,495.41 | $1,624.77 | Agency for Healthcare Research and Quality54 |
| Outpatient | $657.69 | $32.88 | Park and Kuntz55 |
| Adverse events | |||
| Akathisia | $232.94 | $11.65 | Citrome et al19 |
| EPS | $242.01 | $12.10 | US Bureau of Labor Statistics27 |
| Glucose abnormalities | $75.65 | $3.78 | |
| Lipid abnormalities | $173.39 | $8.67 | |
| Sedation | $282.98 | $14.15 | |
| Weight gain ≥7% | $830.19 | $41.51 | |
| Treatment-acquisition costs (WAC) | |||
| Brexpiprazole | $31.16 | $1.56 | Truven Health Analytics56 |
| 4 mg ($/day) | |||
| Lurasidone | |||
| 40 mg ($/day) | $30.73 | $1.54 | |
| 60 mg ($/day) | $30.73 | $1.54 | |
| 80 mg ($/day) | $30.73 | $1.54 | |
| Cariprazine | |||
| 1.5 mg ($/day) | $33.54 | $1.68 | |
| 3.0 mg ($/day) | $33.54 | $1.68 | |
| 4.5 mg ($/day) | $33.54 | $1.68 | |
| 6.0 mg ($/day) | $33.54 | $1.68 | |
| Composite therapy | |||
| Olanzapine | $18.44 | $0.92 | |
| 10 mg ($/day) | |||
| Risperidone | $23.81 | $1.19 | |
| 4 mg ($/day) | |||
| Quetiapine | $18.75 | $0.94 | |
| 200/300 mg ($/day) | |||
| Ziprasidone | $16.24 | $0.81 | |
| 40/60 mg ($/day) | |||
| Aripiprazole | $42.05 | $2.10 | |
| 30 mg ($/day) |
Notes: All costs reported in 2016 US$.
Abbreviations: EPS, extrapyramidal symptoms; WAC, wholesale-acquisition cost.
In the absence of direct-comparison trials of treatments, an indirect comparison was conducted to determine differences in treatment discontinuation and relapse. Because treatments were all compared to placebo in their long-term maintenance trials, this indirect comparison used placebo as the common comparator to obtain model-efficacy values. Derived rates were calculated for treatment discontinuation, which allowed a more accurate comparison of clinical events across comparator cohorts. In general, derived rates were calculated as the product between the relative clinical rate within a trial (active vs respective placebo) and a pooled placebo clinical rate (see Supplementary material for calculation details). The probabilities of relapse at 6 months for treatments in composite therapy are presented in Table 1. Because relapses vary in severity, the model assumed that 77.3% of relapses resulted in an inpatient hospitalization and 22.7% were treated on an outpatient basis.22
Adverse events
The model assumed that patients could experience six types of potential treatment-emergent AEs: akathisia, extrapyramidal symptoms, glucose abnormalities (fasting glucose criteria), lipid abnormalities (fasting total cholesterol criteria), sedation, and weight gain (≥7% weight gain from baseline; Table 2). AE rates were pooled as needed,23 and absolute rates of AEs across comparator trials were used.24,25 AE rates for composite-therapy treatments were obtained from the product labels. Sedation as a unique AE identifier was not reported in the lurasidone PI; therefore, a weighted average was calculated using published data.26
Economic inputs
Cost parameters were derived from the literature, and included schizophrenia-care costs related to drug acquisition, relapse, treatment discontinuation/switching, and treatment costs for AEs (Table 3). Costs are reported as 2016 US$, and where applicable are inflated to 2016 US$ using the medical care component of the consumer price index.27 The model also included the cost of treatment-related monitoring, considered the office-visit cost of monitoring per outpatient,27 and assumed that a treated patient would require one monitoring visit per month. An additional cost of switching treatments was also applied.19,27 If patients discontinued treatment due to other reasons, the analysis did not assign any composite therapy, and thus no additional treatment-related costs applied. Lastly, the costs of treating relevant AEs (Table 3) were assumed to occur only within a 6-week period.
Utility inputs
The model used health-state utilities to estimate the impact of treatments on patients’ QoL. Utility weights were obtained from published QoL data among patients with stable schizophrenia.28,29 Utilities associated with relapse with or without hospitalization and AEs were derived from the utility value from stable schizophrenia. Mean (SE) health-state-utility values were 0.88 (4.4%) for stable disease, 0.53 (2.7%) for relapse with hospitalization, and 0.74 (3.7%) for relapse without hospitalization.28,29 Mean (SE) utility decrements associated with AEs were 0.090 (0.005) for akathisia,28 0.099 (0.005) for extrapyramidal symptoms,28 0.067 (0.003) for glucose abnormalities,30 0.099 (0.005) for lipid abnormalities,31 0.084 (0.004) for sedation,32 and 0.036 (0.002) for weight gain ≥7%.32 Because utility-weight decrements were not available for glucose abnormalities, the utility for symptomatic nonsevere hypoglycemia in patients with diabetes was used.
Analysis
Total direct schizophrenia-related health-care costs, incremental costs, and clinical improvement (ie, number of relapses and hospitalizations avoided) were estimated for each treatment in the model. Incremental cost: effectiveness ratios (ICERs) were expressed as cost per relapse avoided, cost per hospitalization avoided, and cost per QALY gained. These outcomes were calculated at the end of 1 year as the ratio of the difference between the cost of schizophrenia-related care in patients receiving brexpiprazole vs alternative treatment and the difference in the number of patients avoiding relapses or hospitalizations, respectively.
Sensitivity analyses
One-way sensitivity analyses were conducted to quantify the impact of change in individual model parameters on model outcomes. All clinical and economic parameters were varied by 1 SD within a predefined statistical distribution of the base-case values to determine which variables would have the greatest impact on the incremental net monetary benefit (NMB).
To assess uncertainty in the cost-effectiveness analysis, a probabilistic sensitivity analysis (PSA) was also conducted using a second-order Monte Carlo simulation. The PSA was performed by simultaneously drawing from appropriate distribution functions for all model parameters according to their means and SEs (Tables 1–3). All rates were varied using β-distribution and costs varied using γ-distribution. The PSA was repeated 1,000 times, and results reporting the NMB for different willingness-to-pay (WTP) thresholds ($0–$100,000) per selected outcome (avoided relapse, avoided hospitalization, and QALYs) were used to evaluate the robustness of model outcomes.
Scenario analyses were conducted to understand further the impact of model estimate assumptions for AEs and drug costs related to generic options in composite therapy. AE rates were incorporated into the model using absolute estimates from comparator trials. To assess the impact of using derived rates of AEs in the model, a scenario analysis was conducted. Wholesale-acquisition-cost branded pricing was used to estimate drug costs for treatments in the composite-therapy arm. However, given that treatments are generic, a second-scenario analysis using retail pricing from national wholesaler Costco33 was deemed appropriate to assess the impact of lower-cost drug costs.
Results
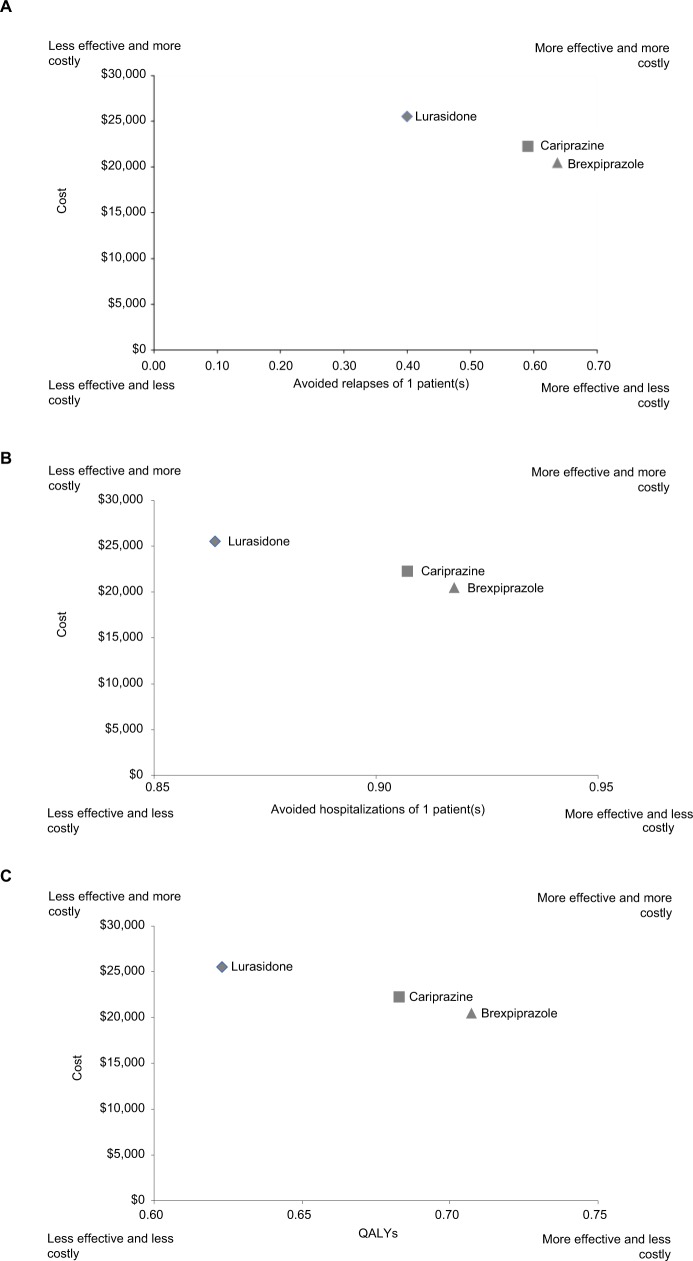
In a hypothetical cohort of 1,000 patients, the model estimated that brexpiprazole was the dominant treatment strategy compared to cariprazine and lurasidone over the 1-year time horizon (Table 4). In terms of clinical outcomes, treatment with brexpiprazole was associated with higher effectiveness (all outcomes shown per patient; avoided relapses 0.637, avoided hospitalizations 0.719, QALYs 0.707), followed by cariprazine (avoided relapses 0.590, avoided hospitalizations 0.683, QALYs 0.683) and lurasidone (avoided relapses 0.400, avoided hospitalizations 0.536, QALYs 0.623). Brexpiprazole was also associated with lower total schizophrenia-related health-care costs per patient ($20,510), followed by cariprazine ($22,282) and lurasidone ($25,510). In the ICE analyses, brexpiprazole was the dominant (ie, less costly and more effective) treatment strategy compared with lurasidone and cariprazine for all ICERs (Table 4). A cost-effectiveness plane displaying results is presented in Figure 2.
Table 4.
Base-case cost-effectiveness analysis
| Brexpiprazole | Lurasidone | Cariprazine | |
|---|---|---|---|
| Total annual cost per patient (medical + pharmacy) | $20,510 | $25,510 | $22,283 |
| Relapses | 0.363 | 0.600 | 0.410 |
| Relapses avoided | 0.637 | 0.400 | 0.590 |
| Hospitalizations | 0.281 | 0.464 | 0.317 |
| Hospitalizations avoided | 0.719 | 0.536 | 0.683 |
| QALYs | 0.707 | 0.623 | 0.683 |
| Change in total cost | −$1,772 | $3,227 | Reference |
| Change in avoided relapses | 0.047 | −0.191 | Reference |
| Change in hospitalizations avoided | 0.036 | −0.147 | Reference |
| Change in QALYs | 0.025 | −0.060 | Reference |
| ICER per avoided relapse | Dominant | Dominated | Reference |
| ICER per hospitalization avoided | Dominant | Dominated | Reference |
| ICER per QALY | Dominant | Dominated | Reference |
Abbreviations: ICER, incremental cost: effectiveness ratio; QALY, quality-adjusted life-year.
Figure 2.
Cost-effectiveness plane per patient.
Notes: (A) Cost-effectiveness per relapse avoided; (B) cost-effectiveness per hospitalization avoided; (C) cost-effectiveness per QALY gained.
Abbreviation: QALY, quality-adjusted life-year.
Sensitivity analyses
Figures S1 and S2 show the results of the one-way sensitivity analyses comparing brexpiprazole vs lurasidone and cariprazine, respectively, for the ten most influential variables at a WTP threshold of $30,000 per relapse avoided. As shown in the tornado diagram, when brexpiprazole was compared to lurasidone (Figure S1), the model parameters with the largest impact on the incremental NMB were the 6-month discontinuation probability due to relapse for brexpiprazole, 6-month discontinuation probability due to relapse for lurasidone, and daily cost of brexpiprazole. Results of one-way sensitivity analyses with the same WTP ($30,000) per relapse-related hospitalization avoided and QALYs showed similar results. When brexpiprazole was compared to cariprazine (Figure S2), the results were consistent with the comparison of brexpiprazole and lurasidone. Results of the PSA using a WTP range of $0–$100,000 per avoided relapse, per avoided hospitalization, and per QALY are shown in Table S1. Based on 1,000 simulations, all results indicated that brexpiprazole was associated with lower cost and better effectiveness, yielding the highest NMB among all comparators.
Scenario analysis
Calculated derived rates of AEs from the first-scenario analysis are presented in Table S2. In this scenario, brexpiprazole was also the dominant treatment strategy compared with lurasidone and cariprazine for all ICERs evaluated (Table S3). In the second-scenario analysis using retail pricing from Costco33 for the composite treatments, cost-effectiveness results were similar to the base-case analysis (Table S4). For each treatment, the total annual cost per patient was slightly lower compared to results reported in the base-case scenario. Results from these two scenario analyses showed consistent findings with the base-case analyses.
Discussion
Schizophrenia poses substantial human and economic burden, and despite the availability of several SGAs, it remains a difficult disorder to treat effectively. A recent study34 found that schizophrenia was one of the three most burdensome diseases on an annual per patient basis, estimated at $46,537 in 2014 US$. Relapses have a significant impact on the economic burden of schizophrenia, with relapsed patients incurring three to four times higher health-care costs than nonrelapsed patients, driven primarily by the costs of hospitalization for relapsed patients.4,35
Brexpiprazole is a recently approved SGA treatment option for adults with schizophrenia. To our knowledge, this is the first cost-effectiveness analysis to compare brexpiprazole with other branded SGAs in reducing schizophrenia relapses and hospitalizations. Results of the base-case cost-effectiveness analyses showed that brexpiprazole was the dominant treatment strategy compared with lurasidone and cariprazine for all outcomes assessed. Although this is the only cost-effectiveness analysis of brexpiprazole compared to lurasidone and cariprazine we are aware of, the cost-effectiveness of lurasidone has been explored in previous studies;36–38 however, these models included different populations, comparators, inputs, assumptions, and time horizons, making comparison across studies difficult. Model results should be considered in light of limitations. The analysis was based on data from placebo-controlled trials; as such, results of this analysis may not be generalizable to treatment provided under real-world conditions. In addition, the probability of relapse for the composite therapy risperidone was not derived from a randomized, placebo-controlled withdrawal study, as one was not conducted by the manufacturer. Therefore, probability of relapse came from a maintenance study of risperidone vs haloperidol that did not involve a period of stabilization followed by medication withdrawal.39
The use of observed AE rates from short-term acute-schizophrenia trials due to lack of long-term comparable comparator data could be another study limitation. However, we employed an indirect-comparison method and derived placebo-adjusted rates in scenario analyses. Both scenario and sensitivity analyses suggested that those rates were not identified as major model drivers and had only a minimal impact on the cost-effectiveness results. Furthermore, incorporation of the short-term cost of treating AEs, such as change in glucose, cholesterol, and weight only reflects short-term treatment costs; however, the potential long-term risks of diabetes, obesity, and complications, such as cerebrovascular accident and cardiovascular disease, are not included in the analysis and warrant consideration in a longer-term evaluation.
The model included only the treatment doses that were evaluated in the long-term prevention trials; therefore, efficacy at higher doses for lurasidone was not evaluated and may affect findings. In the long-term, placebo-controlled maintenance trial of lurasidone, patients were randomized to 40–80 mg/day lurasidone or placebo.21 However, the PI for lurasidone recommends a dose of up to 160 mg per day,25 and some patients who have an inadequate response to doses up to 80 mg/day will require higher-dose treatment.40 As noted by Citrome,41 the Tandon et al21 study had some different findings between US and non-US study sites, which may have further limited the effect size observed in this study relative to other similar studies of SGAs. Furthermore, given the objective of the study, this analysis considered only the branded agents that are available in the US for which a generic formulation is not available and where supportive long-term prevention trials have been published.
Finally, this cost-effectiveness analysis takes a US payer perspective, and thus results may not be generalizable to other populations and/or countries in which health-care-resource utilization and clinical practice may be different. This model also assumed that 77.3% of all relapses resulted in an inpatient hospitalization and the remaining relapses (22.7%) were treated on an outpatient basis.22 This assumption was based on a study conducted in England, which may not reflect US treatment patterns. To account for the impact of various parameter estimates on the model results, we conducted both deterministic and probabilistic sensitivity analyses, which showed consistent results that brexpiprazole dominated lurasidone and cariprazine. As noted by Meltzer, attention is essential to important methodological issues in constructing cost-effectiveness analysis of treatments in schizophrenia.42 There are key issues in developing a cost-effectiveness model in schizophrenia that includes perspective, benefits, and future costs. Due to limited available long-term data across comparators, the current model framework was deemed appropriate to evaluate short-term relapse outcomes.
Conclusion
These findings suggest that treatment with brexpiprazole may lead to clinical benefits and medical cost savings. Brexpiprazole treatment resulted in fewer relapses and hospitalizations, lower total cost of treatment, and higher QoL compared to cariprazine and lurasidone. Given the heterogeneity of treatment response in schizophrenia, health plans may consider making multiple treatment options available, and brexpiprazole offers a cost-effective treatment option.
Supplementary materials
One-way sensitivity analysis for avoided relapses using $30,000 as the WTP threshold (brexpiprazole vs lurasidone)
Abbreviations: WAC, whole acquisition cost; WTP, willingness to pay.
One-way Sensitivity analysis for avoided relapses using $30,000 as the WTP threshold (brexpiprazole vs cariprazine)
Abbreviations: WAC, whole acquisition cost; WTP, willingness to pay.
Table S1.
Mean probabilistic sensitivity analysis results
| Outcome: relapses avoided per patient
| ||||
|---|---|---|---|---|
| Total annual cost per patient (medical + pharmacy costs) | Relapses avoided per patient | Net monetary benefit | Rank | |
| Brexpiprazole | $20,457 | 0.707 | $50,283 | 1 |
| Cariprazine | $22,254 | 0.683 | $46,021 | 2 |
| Lurasidone | $25,457 | 0.623 | $36,814 | 3 |
|
| ||||
|
Outcome: relapse-related hospitalization avoided per patient
| ||||
| Total annual cost per patient (medical + pharmacy costs) | Relapse-related hospitalization avoided per patient | Net monetary benefit | Rank | |
|
| ||||
| Brexpiprazole | $18,940 | 0.719 | $52,978 | 1 |
| Cariprazine | $21,200 | 0.683 | $47,143 | 2 |
| Lurasidone | $23,929 | 0.536 | $29,663 | 3 |
|
| ||||
|
Outcome: QALYs per patient
| ||||
| Total annual cost per patient (medical + pharmacy costs) | QALYs per patient | Net monetary benefit | Rank | |
|
| ||||
| Brexpiprazole | $20,504 | 0.706 | $50,146 | 1 |
| Cariprazine | $22,311 | 0.683 | $45,894 | 2 |
| Lurasidone | $25,549 | 0.623 | $36,706 | 3 |
Abbreviation: QALY, quality-adjusted life-year.
Table S2.
Scenario analysis: derived rates of adverse events
| Treatment | Akathisia mean | EPS mean | Glucose abnormalitiesa mean | Lipid abnormalitiesb mean | Sedation mean | Weight gain ≥7% mean | Source |
|---|---|---|---|---|---|---|---|
| Brexpiprazole | 5.56% | 11.88% | 6.82% | 4.21% | 11.33% | 6.63% | Rexulti PI1 |
| Lurasidone | 14.24% | 13.67% | 7.66% | 4.55% | 7.77% | 3.87% | Latuda PI2 Citrome 20123 |
| Cariprazine | 10.19% | 14.82%c | 8.14% | 3.85% | 3.36%d | 7.99% | Vraylar Medical Reviews4 Durgam 20145 |
| Composite therapy | 7.8% | 13.4% | 2.7% | 4.2% | 13.8% | 21.2% | Calculation (mean rate across SGAs) |
| Olanzapine | 4.52% | 15.19% | 1.70% | 3.09% | 10.72%e | 38.48% | Zyprexa PI6 |
| Risperidone | 17.34% | 18.93%f | 1.51% | 4.50% | 23.68% | 19.33% | Risperdal PI7 |
| Quetiapine | 0.79% | 4.53%g | 4.52% | 7.12% | 12.93%e | 22.17% | Seroquel PI8 |
| Ziprasidone | 5.94% | 17.60% | 3.01% | 3.95% | 11.49%e | 12.55% | Geodon PI9 |
| Aripiprazole | 10.40% | 10.90% | 2.78% | 2.37% | 10.05%h | 13.30% | Abilify PI10 |
Notes: Rates of adverse events are absolute rates (not placebo-adjusted).
Fasting glucose criteria: Normal to high (<100 to >126 mg/dL). For lurasidone, the normal to high criterion was ≥160 mg/dL; for risperidone, the normal to high cutoff was <140 mg/dL to ≥200 mg/dL.
Fasting total cholesterol criteria: Normal to high (<200 mg/dL to ≥240 mg/dL). For lurasidone, the normal to high criterion was <200 mg/dL; for quetiapine, the criterion was ≥240 mg/dL.
Any EPS excluding akathisia/restlessness.
Weighted average was calculated based on total cholesterol (>1.3 times ULN [200 mg/dL]) rates from 3 treatment arms: 1.5 mg, 3 mg, and 4.5 mg.
No sedation data were reported. Somnolence rate was used.
No EPS data were reported. Parkinsonism (includes extrapyramidal disorder, musculoskeletal stiffness, parkinsonism, cogwheel rigidity, akinesia, bradykinesia, hypokinesia) rate from 2 treatment arms: 2–8 mg and >8–16 mg/day.
Includes restless and extrapyramidal disorder.
Data from pooled incidence, rounded to the nearest percent, of adverse reactions that occurred during acute therapy (up to 6 weeks in schizophrenia and up to 3 weeks in bipolar mania).
Abbreviations: AE, adverse event; EPS, extrapyramidal symptoms; PI, package insert; SGA, second-generation antipsychotic; ULN, upper limit of normal.
Table S3.
Scenario analysis: results of cost-effectiveness analysis using derived AE rates
| Brexpiprazole | Lurasidone | Cariprazine | |
|---|---|---|---|
| Total annual cost per patient (medical + pharmacy costs) | $20,516 | $25,519 | $22,349 |
| Relapses | 0.363 | 0.600 | 0.410 |
| Avoided relapses | 0.637 | 0.400 | 0.590 |
| Hospitalizations | 0.281 | 0.464 | 0.317 |
| Avoided hospitalizations | 0.719 | 0.536 | 0.683 |
| QALYs | 0.698 | 0.624 | 0.684 |
| Change in total cost | $1,833 | $3,170 | Reference |
| Change in avoided relapses | 0.047 | −0.191 | Reference |
| Change in avoided hospitalizations | 0.036 | −0.147 | Reference |
| Change in QALYs | 0.014 | −0.060 | Reference |
| ICER per avoided relapse | Dominant | Dominated | Reference |
| ICER per avoided hospitalization | Dominant | Dominated | Reference |
| ICER per QALY | Dominant | Dominated | Reference |
Abbreviations: AE, adverse event; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year.
Table S4.
Scenario analysis: results of cost-effectiveness analysis using generic drug costs for treatments in composite therapy arm
| Brexpiprazole | Lurasidone | Cariprazine | |
|---|---|---|---|
| Total annual cost per patient (medical + pharmacy costs) | $18,931 | $23,955 | $21,210 |
| Relapses | 0.363 | 0.600 | 0.410 |
| Avoided relapses | 0.637 | 0.400 | 0.590 |
| Hospitalizations | 0.281 | 0.464 | 0.317 |
| Avoided hospitalizations | 0.719 | 0.536 | 0.683 |
| QALYs | 0.707 | 0.623 | 0.683 |
| Change in total cost | −$2,279 | $2,745 | Reference |
| Change in avoided relapses | 0.0467 | −0.191 | Reference |
| Change in avoided hospitalizations | 0.036 | −0.147 | Reference |
| Change in QALYs | 0.0245 | −0.060 | Reference |
| ICER per avoided relapse | Dominant | Dominated | Reference |
| ICER per avoided hospitalization | Dominant | Dominated | Reference |
| ICER per QALY | Dominant | Dominated | Reference |
Abbreviations: ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year.
References
- 1.Rexulti [package insert] Tokyo: Otsuka Pharmaceutical; 2016. [Google Scholar]
- 2.Latuda [package insert] Marlborough (MA): Sunovion Pharmaceuticals; 2013. [Google Scholar]
- 3.Citrome L. Brexpiprazole for schizophrenia and as adjunct for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antipsychotic – what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2015;69(9):978–997. doi: 10.1111/ijcp.12714. [DOI] [PubMed] [Google Scholar]
- 4.Vraylar (cariprazine) [summary review] 2015. [Accessed June 19, 2018]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/204370Orig1Orig2s000SumR.pdf.
- 5.Durgam S, Starace A, Li D, et al. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trial. Schizophr Res. 2014;152(2–3):450–457. doi: 10.1016/j.schres.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 6.Zyprexa [package insert] Indianapolis: Lilly USA; 2014. [Google Scholar]
- 7.Risperdal [package insert] Titusville (NJ): Janssen Pharmaceutical; 2009. [Google Scholar]
- 8.Seroquel [package insert] Wilmington (DE): AstraZeneca Pharmaceuticals; 2009. [Google Scholar]
- 9.Geodon [package insert] New York: Pfizer; 2009. [Google Scholar]
- 10.Abilify [package insert] Tokyo: Otsuka Pharmaceutical; 2012. [Google Scholar]
- 11.Fleischhacker WW, Hobart M, Ouyang J, et al. Efficacy and safety of brexpiprazole (OPC-34712) as maintenance treatment in adults with schizophrenia: a randomised, double-blind, placebo-controlled study. Int J Neuropsychopharmacol. 2017;20(1):11–21. doi: 10.1093/ijnp/pyw076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Acknowledgments
Editorial support for the preparation of this manuscript was provided by Ann Cameron, PhD of Xcenda LLC, and funded by Otsuka America Pharmaceutical Inc. Princeton, NJ, USA, and Lundbeck LLC, Deerfield, IL, USA. She has no conflicts of interest to declare. This work was presented as a poster at the 28th Annual US Psychiatric and Mental Health Congress, San Diego, CA, September 10–13, 2015.
Appendix 1
Calculation of treatment discontinuation relative risks
Treatment discontinuation relative risks were calculated for active treatment vs placebo using a 3-step method. First, patients who were terminated by the sponsor were removed from the efficacy sample, and the discontinuation rate due to relapse (or due to AE or other reasons) was recalculated for both the treatment and placebo groups. Of note, in the maintenance trial of cariprazine, no patients were terminated by the sponsor; therefore, this step was skipped for the cariprazine calculation.11 Next, to ensure that all probabilities were calculated within the same time frame, any transition probabilities other than 6 months were converted by using the following formula where EXP refers to the exponential function and LN refers to the natural logarithm function: 1-EXP(LN(1-Probability)/(Number of weeks in the original trial/26)). Finally, the 6-month probability from step 2 was adjusted by applying the relative risk method where the product between the relative clinical rate within trial (active vs respective placebo) and a pooled placebo clinical rate was calculated.
Footnotes
Disclosure
MSA is an employee at Otsuka Pharmaceutical Development and Commercialization, Inc. SAK was a full time employee of Otsuka Pharmaceutical Development and Commercialization, Inc at the time of this research. In the past 36 months, LC has engaged in collaborative research with or received consulting or speaking fees from Acadia, Alexza, Alkermes, Allergan, AstraZeneca, Avanir, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Forum, Genentech, Janssen, Jazz, Lundbeck, Merck, Medivation, Mylan, Neurocrine, Novartis, Noven, Otsuka, Pfizer, Reckitt Benckiser, Reviva, Shire, Sunovion, Takeda, Teva, Valeant, and Vanda. AMD and SL are employees of Xcenda, a consulting company that received funds from Otsuka America Pharmaceutical and Lundbeck LLC to conduct this study. The authors report no other conflicts of interest in this work.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington (VA): APA; 2013. [Google Scholar]
- 2.National Institute of Mental Health Schizophrenia. 2016. [Accessed June 19, 2018]. Available from: http://www.nimh.nih.gov/health/statistics/prevalence/schizophrenia.shtml.
- 3.Cloutier M, Aigbogun MS, Guerin A, et al. The economic burden of schizophrenia in the United States in 2013. J Clin Psychiatry. 2016;77(6):764–771. doi: 10.4088/JCP.15m10278. [DOI] [PubMed] [Google Scholar]
- 4.Ascher-Svanum H, Zhu B, Faries DE, et al. The cost of relapse and the predictors of relapse in the treatment of schizophrenia. BMC Psychiatry. 2010;10:2. doi: 10.1186/1471-244X-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volavka J, Citrome L. Oral antipsychotics for the treatment of schizophrenia: heterogeneity in efficacy and tolerability should drive decision-making. Expert Opin Pharmacother. 2009;10(12):1917–1928. doi: 10.1517/14656560903061309. [DOI] [PubMed] [Google Scholar]
- 6.Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 Suppl):1–56. [PubMed] [Google Scholar]
- 7.Brissos S, Molodynski A, Dias VV, Figueira ML. The importance of measuring psychosocial functioning in schizophrenia. Ann Gen Psychiatry. 2011;10:18. doi: 10.1186/1744-859X-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia. 2nd ed. 2010. [Accessed June 19, 2018]. Available from: https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf.
- 9.Emsley R, Chiliza B, Asmal L, Harvey BH. The nature of relapse in schizophrenia. BMC Psychiatry. 2013;13:50. doi: 10.1186/1471-244X-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lieberman JA, Stroup TS, Mcevoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 11.Haro JM, Suarez D, Novick D, Brown J, Usall J, Naber D. Three-year antipsychotic effectiveness in the outpatient care of schizophrenia: observational versus randomized studies results. Eur Neuropsychopharmacol. 2007;17(4):235–244. doi: 10.1016/j.euroneuro.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Ascher-Svanum H, Nyhuis AW, Stauffer V, et al. Reasons for discontinuation and continuation of antipsychotics in the treatment of schizophrenia from patient and clinician perspectives. Curr Med Res Opin. 2010;26(10):2403–2410. doi: 10.1185/03007995.2010.515900. [DOI] [PubMed] [Google Scholar]
- 13.Liu-Seifert H, Adams DH, Kinon BJ. Discontinuation of treatment of schizophrenic patients is driven by poor symptom response: a pooled post-hoc analysis of four atypical antipsychotic drugs. BMC Med. 2005;3:21. doi: 10.1186/1741-7015-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Correll CU, Skuban A, Ouyang J, et al. Efficacy and safety of brexpiprazole for the treatment of acute schizophrenia: a 6-week randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2015;172(9):870–880. doi: 10.1176/appi.ajp.2015.14101275. [DOI] [PubMed] [Google Scholar]
- 15.Kane JM, Skuban A, Ouyang J, et al. A multicenter, randomized, double-blind, controlled phase 3 trial of fixed-dose brexpiprazole for the treatment of adults with acute schizophrenia. Schizophr Res. 2015;164(1–3):127–135. doi: 10.1016/j.schres.2015.01.038. [DOI] [PubMed] [Google Scholar]
- 16.Kane JM, Skuban A, Hobart M, et al. Overview of short- and long-term tolerability and safety of brexpiprazole in patients with schizophrenia. Schizophr Res. 2016;174(1–3):93–98. doi: 10.1016/j.schres.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Fleischhacker WW, Hobart M, Ouyang J, et al. Efficacy and safety of brexpiprazole (OPC-34712) as maintenance treatment in adults with schizophrenia: a randomised, double-blind, placebo-controlled study. Int J Neuropsychopharmacol. 2017;20(1):11–21. doi: 10.1093/ijnp/pyw076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neumann PJ. Methods of cost-effectiveness analysis in the evaluation of new antipsychotics: implications for schizophrenia treatment. J Clin Psychiatry. 1999;60(Suppl 3):9–15. [PubMed] [Google Scholar]
- 19.Citrome L, Kamat SA, Sapin C, et al. Cost-effectiveness of aripiprazole once-monthly compared with paliperidone palmitate once-monthly injectable for the treatment of schizophrenia in the United States. J Med Econ. 2014;17(8):567–576. doi: 10.3111/13696998.2014.917089. [DOI] [PubMed] [Google Scholar]
- 20.Durgam S, Earley W, Li R, et al. Long-term cariprazine treatment for the prevention of relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled trial. Schizophr Res. 2016;176(2–3):264–271. doi: 10.1016/j.schres.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 21.Tandon R, Cucchiaro J, Phillips D, et al. A double-blind, placebo-controlled, randomized withdrawal study of lurasidone for the maintenance of efficacy in patients with schizophrenia. J Psychopharmacol. 2016;30(1):69–77. doi: 10.1177/0269881115620460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Institute for Health and Care Excellence Psychosis and schizophrenia overview. 2018. [Accessed June 19, 2018]. Available from: https://pathways.nice.org.uk/pathways/psychosis-and-schizophrenia.
- 23.Citrome L. Brexpiprazole for schizophrenia and as adjunct for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antipsychotic – what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2015;69(9):978–997. doi: 10.1111/ijcp.12714. [DOI] [PubMed] [Google Scholar]
- 24.Rexulti [package insert] Tokyo: Otsuka Pharmaceutical; 2016. [Google Scholar]
- 25.Latuda [package insert] Marlborough (MA): Sunovion Pharmaceuticals; 2013. [Google Scholar]
- 26.Citrome L. Lurasidone for the acute treatment of adults with schizophrenia: what is the number needed to treat, number needed to harm, and likelihood to be helped or harmed? Clin Schizophr Relat Psychoses. 2012;6(2):76–85. doi: 10.3371/CSRP.6.2.5. [DOI] [PubMed] [Google Scholar]
- 27.US Bureau of Labor Statistics Consumer price index: medical care. [Accessed June 19, 2018]. Available from: https://data.bls.gov/cgi-bin/surveymost?cu.
- 28.Lenert LA, Sturley AP, Rapaport MH, et al. Public preferences for health states with schizophrenia and a mapping function to estimate utilities from positive and negative symptom scale scores. Schizophr Res. 2004;71(1):155–165. doi: 10.1016/j.schres.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Furiak NM, Ascher-Svanum H, Klein RW, et al. Cost-effectiveness of olanzapine long-acting injection in the treatment of patients with schizophrenia in the United States: a micro-simulation economic decision model. Curr Med Res Opin. 2011;27(4):713–730. doi: 10.1185/03007995.2011.554533. [DOI] [PubMed] [Google Scholar]
- 30.Levy AR, Christensen TL, Johnson JA. Utility values for symptomatic non-severe hypoglycaemia elicited from persons with and without diabetes in Canada and the United Kingdom. Health Qual Life Outcomes. 2008;6:73. doi: 10.1186/1477-7525-6-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ascher-Svanum H, Furiak NM, Lawson AH, et al. Cost-effectiveness of several atypical antipsychotics in orally disintegrating tablets compared with standard oral tablets in the treatment of schizophrenia in the United States. J Med Econ. 2012;15(3):531–547. doi: 10.3111/13696998.2012.662923. [DOI] [PubMed] [Google Scholar]
- 32.Treur M, Baca E, Bobes J, et al. The cost-effectiveness of paliperidone extended release in Spain. J Med Econ. 2012;15(Suppl 1):26–34. doi: 10.3111/13696998.2012.734884. [DOI] [PubMed] [Google Scholar]
- 33.Costco [homepage] [Accessed June 19, 2018]. Available from: https://www.costco.com.
- 34.Macewan JP, Seabury S, Aigbogun MS, et al. Pharmaceutical innovation in the treatment of schizophrenia and mental disorders compared with other diseases. Innov Clin Neurosci. 2016;13(7–8):17–25. [PMC free article] [PubMed] [Google Scholar]
- 35.Almond S, Knapp M, Francois C, Toumi M, Brugha T. Relapse in schizophrenia: costs, clinical outcomes and quality of life. Br J Psychiatry. 2004;184:346–351. doi: 10.1192/bjp.184.4.346. [DOI] [PubMed] [Google Scholar]
- 36.Ng-Mak D, Chuang CC, Baker T, Li J, Rajagopalan K, Loebel A. Lurasidone versus brexpiprazole in adult schizophrenia: cost-effectiveness analysis. Poster presented at: 29th Annual US Psychiatric and Mental Health Congress; October 21–24; San Antonio, TX. [Google Scholar]
- 37.O’Day K, Rajagopalan K, Meyer K, Pikalov A, Loebel A. Long-term cost-effectiveness of atypical antipsychotics in the treatment of adults with schizophrenia in the US. Clinicoecon Outcomes Res. 2013;5:459–470. doi: 10.2147/CEOR.S47990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajagopalan K, Hassan M, O’Day K, Meyer K, Grossman F. Cost-effectiveness of lurasidone vs aripiprazole among patients with schizophrenia who have previously failed on an atypical antipsychotic: an indirect comparison of outcomes from clinical trial data. J Med Econ. 2013;16(7):951–961. doi: 10.3111/13696998.2013.807813. [DOI] [PubMed] [Google Scholar]
- 39.Csernansky JG, Mahmoud R, Brenner R. A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med. 2002;346(1):16–22. doi: 10.1056/NEJMoa002028. [DOI] [PubMed] [Google Scholar]
- 40.Loebel A, Silva R, Goldman R, et al. Lurasidone dose escalation in early nonresponding patients with schizophrenia: a randomized, placebo-controlled study. J Clin Psychiatry. 2016;77(12):1672–1680. doi: 10.4088/JCP.16m10698. [DOI] [PubMed] [Google Scholar]
- 41.Citrome L. Schizophrenia relapse, patient considerations, and potential role of lurasidone. Patient Prefer Adherence. 2016;10:1529–1537. doi: 10.2147/PPA.S45401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meltzer D. Perspective and the measurement of costs and benefits for cost-effectiveness analysis in schizophrenia. J Clin Psychiatry. 1999;60(Suppl 33):32–37. [PubMed] [Google Scholar]
- 43.Beasley CM, Sutton VK, Hamilton SH, et al. A double-blind, randomized, placebo-controlled trial of olanzapine in the prevention of psychotic relapse. J Clin Psychopharmacol. 2003;23(6):582–594. doi: 10.1097/01.jcp.0000095348.32154.ec. [DOI] [PubMed] [Google Scholar]
- 44.Peuskens J, Trivedi J, Malyarov S, et al. Prevention of schizophrenia relapse with extended release quetiapine fumarate dosed once daily: a randomized, placebo-controlled trial in clinically stable patients. Psychiatry (Edgmont) 2007;4(11):34–50. [PMC free article] [PubMed] [Google Scholar]
- 45.Arato M, O’Connor R, Meltzer HY. A 1-year, double-blind, placebo-controlled trial of ziprasidone 40, 80 and 160 mg/day in chronic schizophrenia: the Ziprasidone Extended Use in Schizophrenia (ZEUS) study. Int Clin Psychopharmacol. 2002;17(5):207–215. doi: 10.1097/00004850-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Pigott TA, Carson WH, Saha AR, et al. Aripiprazole for the prevention of relapse in stabilized patients with chronic schizophrenia: a placebo-controlled 26-week study. J Clin Psychiatry. 2003;64(9):1048–1056. doi: 10.4088/jcp.v64n0910. [DOI] [PubMed] [Google Scholar]
- 47.Vraylar (cariprazine) [summary review] 2015. [Accessed June 19, 2018]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/204370Orig1Orig2s000SumR.pdf.
- 48.Durgam S, Starace A, Li D, et al. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trial. Schizophr Res. 2014;152(2–3):450–457. doi: 10.1016/j.schres.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 49.Zyprexa [package insert] Indianapolis: Lilly USA; 2014. [Google Scholar]
- 50.Risperdal [package insert] Titusville (NJ): Janssen Pharmaceutical; 2009. [Google Scholar]
- 51.Seroquel [package insert] Wilmington (DE): AstraZeneca Pharmaceuticals; 2009. [Google Scholar]
- 52.Geodon [package insert] New York: Pfizer; 2009. [Google Scholar]
- 53.Abilify [package insert] Tokyo: Otsuka Pharmaceutical; 2012. [Google Scholar]
- 54.Agency for Healthcare Research and Quality HCUPnet: Healthcare Cost and Utilization Project Nationwide Inpatient Sample. 2013. [Accessed June 19, 2018]. Available from: http://hcupnet.ahrq.gov.
- 55.Park T, Kuntz KM. Cost-effectiveness of second-generation antipsychotics for the treatment of schizophrenia. Value Health. 2014;17(4):310–319. doi: 10.1016/j.jval.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 56.Truven Health Analytics . Micromedex 2.0: introduction to Red Book online. [Accessed June 19, 2018]. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
One-way sensitivity analysis for avoided relapses using $30,000 as the WTP threshold (brexpiprazole vs lurasidone)
Abbreviations: WAC, whole acquisition cost; WTP, willingness to pay.
One-way Sensitivity analysis for avoided relapses using $30,000 as the WTP threshold (brexpiprazole vs cariprazine)
Abbreviations: WAC, whole acquisition cost; WTP, willingness to pay.
Table S1.
Mean probabilistic sensitivity analysis results
| Outcome: relapses avoided per patient
| ||||
|---|---|---|---|---|
| Total annual cost per patient (medical + pharmacy costs) | Relapses avoided per patient | Net monetary benefit | Rank | |
| Brexpiprazole | $20,457 | 0.707 | $50,283 | 1 |
| Cariprazine | $22,254 | 0.683 | $46,021 | 2 |
| Lurasidone | $25,457 | 0.623 | $36,814 | 3 |
|
| ||||
|
Outcome: relapse-related hospitalization avoided per patient
| ||||
| Total annual cost per patient (medical + pharmacy costs) | Relapse-related hospitalization avoided per patient | Net monetary benefit | Rank | |
|
| ||||
| Brexpiprazole | $18,940 | 0.719 | $52,978 | 1 |
| Cariprazine | $21,200 | 0.683 | $47,143 | 2 |
| Lurasidone | $23,929 | 0.536 | $29,663 | 3 |
|
| ||||
|
Outcome: QALYs per patient
| ||||
| Total annual cost per patient (medical + pharmacy costs) | QALYs per patient | Net monetary benefit | Rank | |
|
| ||||
| Brexpiprazole | $20,504 | 0.706 | $50,146 | 1 |
| Cariprazine | $22,311 | 0.683 | $45,894 | 2 |
| Lurasidone | $25,549 | 0.623 | $36,706 | 3 |
Abbreviation: QALY, quality-adjusted life-year.
Table S2.
Scenario analysis: derived rates of adverse events
| Treatment | Akathisia mean | EPS mean | Glucose abnormalitiesa mean | Lipid abnormalitiesb mean | Sedation mean | Weight gain ≥7% mean | Source |
|---|---|---|---|---|---|---|---|
| Brexpiprazole | 5.56% | 11.88% | 6.82% | 4.21% | 11.33% | 6.63% | Rexulti PI1 |
| Lurasidone | 14.24% | 13.67% | 7.66% | 4.55% | 7.77% | 3.87% | Latuda PI2 Citrome 20123 |
| Cariprazine | 10.19% | 14.82%c | 8.14% | 3.85% | 3.36%d | 7.99% | Vraylar Medical Reviews4 Durgam 20145 |
| Composite therapy | 7.8% | 13.4% | 2.7% | 4.2% | 13.8% | 21.2% | Calculation (mean rate across SGAs) |
| Olanzapine | 4.52% | 15.19% | 1.70% | 3.09% | 10.72%e | 38.48% | Zyprexa PI6 |
| Risperidone | 17.34% | 18.93%f | 1.51% | 4.50% | 23.68% | 19.33% | Risperdal PI7 |
| Quetiapine | 0.79% | 4.53%g | 4.52% | 7.12% | 12.93%e | 22.17% | Seroquel PI8 |
| Ziprasidone | 5.94% | 17.60% | 3.01% | 3.95% | 11.49%e | 12.55% | Geodon PI9 |
| Aripiprazole | 10.40% | 10.90% | 2.78% | 2.37% | 10.05%h | 13.30% | Abilify PI10 |
Notes: Rates of adverse events are absolute rates (not placebo-adjusted).
Fasting glucose criteria: Normal to high (<100 to >126 mg/dL). For lurasidone, the normal to high criterion was ≥160 mg/dL; for risperidone, the normal to high cutoff was <140 mg/dL to ≥200 mg/dL.
Fasting total cholesterol criteria: Normal to high (<200 mg/dL to ≥240 mg/dL). For lurasidone, the normal to high criterion was <200 mg/dL; for quetiapine, the criterion was ≥240 mg/dL.
Any EPS excluding akathisia/restlessness.
Weighted average was calculated based on total cholesterol (>1.3 times ULN [200 mg/dL]) rates from 3 treatment arms: 1.5 mg, 3 mg, and 4.5 mg.
No sedation data were reported. Somnolence rate was used.
No EPS data were reported. Parkinsonism (includes extrapyramidal disorder, musculoskeletal stiffness, parkinsonism, cogwheel rigidity, akinesia, bradykinesia, hypokinesia) rate from 2 treatment arms: 2–8 mg and >8–16 mg/day.
Includes restless and extrapyramidal disorder.
Data from pooled incidence, rounded to the nearest percent, of adverse reactions that occurred during acute therapy (up to 6 weeks in schizophrenia and up to 3 weeks in bipolar mania).
Abbreviations: AE, adverse event; EPS, extrapyramidal symptoms; PI, package insert; SGA, second-generation antipsychotic; ULN, upper limit of normal.
Table S3.
Scenario analysis: results of cost-effectiveness analysis using derived AE rates
| Brexpiprazole | Lurasidone | Cariprazine | |
|---|---|---|---|
| Total annual cost per patient (medical + pharmacy costs) | $20,516 | $25,519 | $22,349 |
| Relapses | 0.363 | 0.600 | 0.410 |
| Avoided relapses | 0.637 | 0.400 | 0.590 |
| Hospitalizations | 0.281 | 0.464 | 0.317 |
| Avoided hospitalizations | 0.719 | 0.536 | 0.683 |
| QALYs | 0.698 | 0.624 | 0.684 |
| Change in total cost | $1,833 | $3,170 | Reference |
| Change in avoided relapses | 0.047 | −0.191 | Reference |
| Change in avoided hospitalizations | 0.036 | −0.147 | Reference |
| Change in QALYs | 0.014 | −0.060 | Reference |
| ICER per avoided relapse | Dominant | Dominated | Reference |
| ICER per avoided hospitalization | Dominant | Dominated | Reference |
| ICER per QALY | Dominant | Dominated | Reference |
Abbreviations: AE, adverse event; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year.
Table S4.
Scenario analysis: results of cost-effectiveness analysis using generic drug costs for treatments in composite therapy arm
| Brexpiprazole | Lurasidone | Cariprazine | |
|---|---|---|---|
| Total annual cost per patient (medical + pharmacy costs) | $18,931 | $23,955 | $21,210 |
| Relapses | 0.363 | 0.600 | 0.410 |
| Avoided relapses | 0.637 | 0.400 | 0.590 |
| Hospitalizations | 0.281 | 0.464 | 0.317 |
| Avoided hospitalizations | 0.719 | 0.536 | 0.683 |
| QALYs | 0.707 | 0.623 | 0.683 |
| Change in total cost | −$2,279 | $2,745 | Reference |
| Change in avoided relapses | 0.0467 | −0.191 | Reference |
| Change in avoided hospitalizations | 0.036 | −0.147 | Reference |
| Change in QALYs | 0.0245 | −0.060 | Reference |
| ICER per avoided relapse | Dominant | Dominated | Reference |
| ICER per avoided hospitalization | Dominant | Dominated | Reference |
| ICER per QALY | Dominant | Dominated | Reference |
Abbreviations: ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year.